Research Highlights
3D Printed Tumor Spheroids for Disease Modeling and Chemotherapeutic Drug Screening
Institution:

Florida A&M University
Team:
Aragaw Gebeyehu1,4, Sunil Kumar Surapaneni1,4, John Huang2, Arindam Mondal1, Vivian Ziwen Wang2, Nana Fatima Haruna2, Arvind Bagde1, Peggy Arthur1, Shallu Kutlehria1, Nil Patel1, Arun K. Rishi3 & Mandip Singh1*
Application:
3D bioprint of cancer cell lines to perform drug testings.
Cell types:
NSCLC-PDX, MDA MB231 WT, and HCC B-27 cells
Hydrogel:
VitroINK® 3D (INK01-10) and VitroINK® RGD (INK02-10)
Cancer drug development is typically evaluated in conventional two-dimensional (2D) cell culture platforms. However, conventional 2D cultured cancer cells cannot mimic the complexity and heterogeneity of in-vivo tumors, which usually grow in a three-dimensional (3D) conformation. To overcome this, various 3D cell culture platforms are being currently developed, which can mimic the in-vivo tumor microenvironment as spheroid cells or organoid models. Hydrogel-based 3D scaffolds are gaining attention due to their cell encapsulation capability and property of mimicking native extracellular matrix (ECM). Particularly, bioprinting techniques are interesting in the field of tissue engineering due to their ability to create complex structures. 3D printing of cell-laden scaffolds facilitates homogenous cell/spheroids distribution, homogenous drug diffusion through the entire scaffolds, and opens new avenues for the fabrication of microtissues. However, it is challenging to find a bio-ink material with printability, shape-fidelity, and tunable mechanical properties that maintain cell viability and supports rapid spheroid formation. Moreover, the crosslinking method is also another key parameter of the bio-ink for 3D printed scaffolds fabrication. Toxicity and unwanted reactions of the crosslinker with the bioactive substances present in the hydrogel matrix limit their application. Changes in pH and temperature, high energy radiation, free radical polymerizations can be used for crosslinking in 3D cultures. However, these methods reduce cell viability and compromise the integrity of the cell culture study.
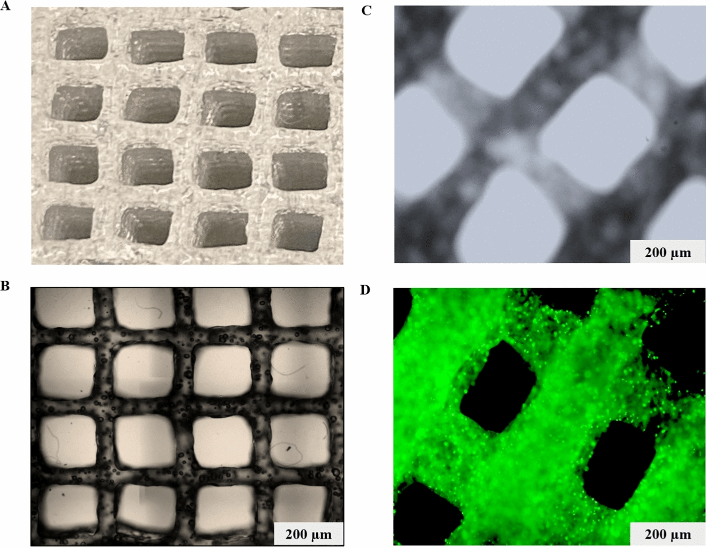
Gebeyehu and his colleagues tested a series of room temperature stable and ready-to-use hydrogels to develop a bioink that mimics the in-vivo tumor microenvironment, shows good printability, rheological characteristics (i.e., shear-thinning, and rapid recovery rheological properties of hydrogels can lead to a stable printed structure after extrusion), biocompatibility and, also can be used for the application of screening of anti-cancer drugs. They observed that VitroINK 3D and VitroINK RGD displayed excellent rheological properties among all screened samples. These bio-inks can be crosslinked with cell culture medium, which facilitates good cell growth and scaffold stability. VitroINK RGD was printable with less cell destructive extrusion pressure and showed more than 90% cell viability for both tumor and normal cells. The constructed cell-laden scaffold maintained its stiffness for more than 20 days of incubation at 37 °C. NSCLC PDX (EGFR T790M) cells, which were cultured in 3D printed Ink H-RGD scaffold, showed high cell proliferation with tumor microenvironment and spheroid formation (500 μm in diameter) within 7 days. IC50 values of docetaxel, doxorubicin, and erlotinib demonstrated higher resistance in 3D spheroids of NSCLC-PDX, MDA MB231 WT, and HCC B-27 cells when compared to 2D monolayers cells, as analyzed by cytotoxicity assay (P <0.001).
Read the publication:
Related Product: