High cell quality
Yields high-quality, functional cells to support full downstream differentiation.
VitroGel® MSC
ready-to-use, xeno-free (animal origin-free) hydrogel system for mesenchymal stem cell 3D culture and scale-up
VitroGel® MSC
Ready-to-use, xeno-free hydrogel for 3D culture and scale-up for mesenchymal stem cell (MSC) and exosome production

High Expansion rate
Supports fast MSC expansion and long-term cell culture

Easy setup and recovery; No-microcarrier needed
Simple, 30-minute set-up. No microcarrier is required, eliminating long hours of seeding, suspension steps, and recovery.
Supports exosome production
Supports 3D bioprocess for exosome production with high purity and high yield.
Easy cell harvesting
Simple and efficient cell harvesting by either centrifuge or the non-enzymatic VitroGel® Organoid Recovery Solution.

Injectable hydrogel
100% xeno-free hydrogel system and ideal for in vitro and in vivo applications.
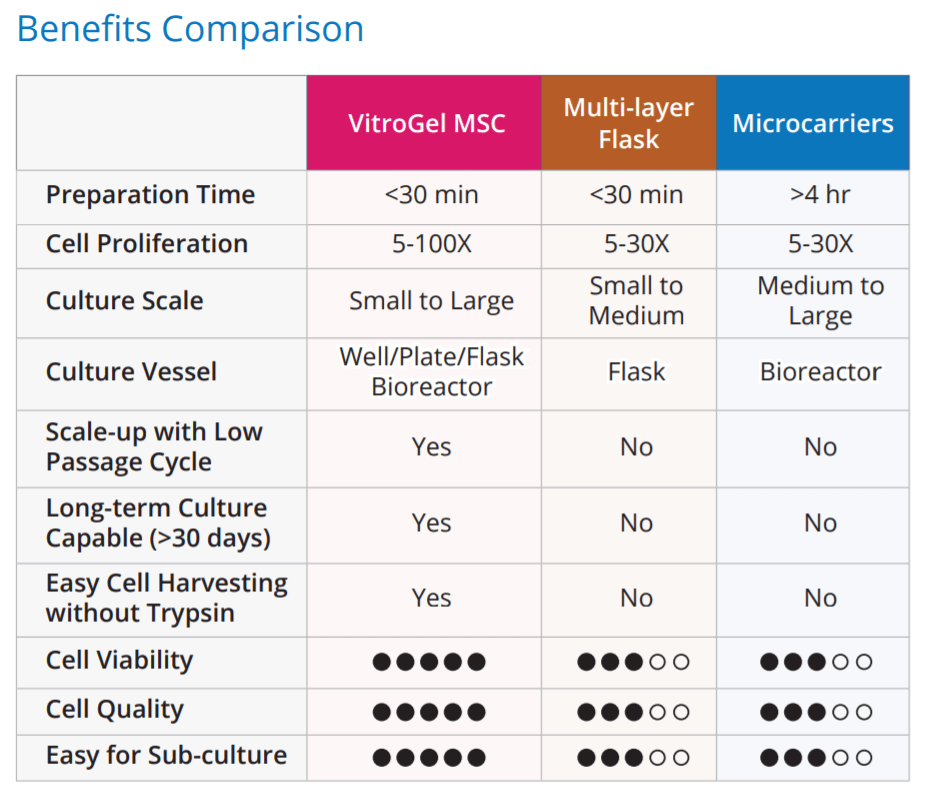
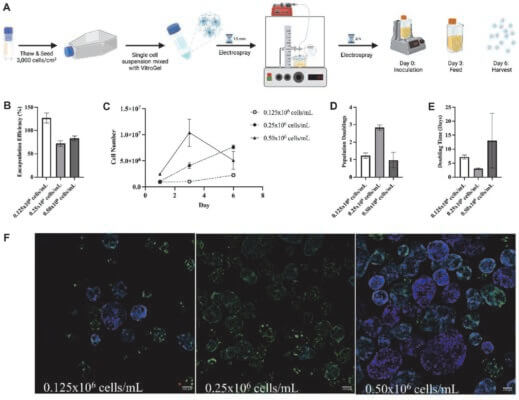
VitroGel® MSC is a xeno-free (animal origin-free) hydrogel system for 3D culture and scale-up of mesenchymal stem cells (MSCs) and exosome production. This hydrogel system can be used to make hydrogel cell beads for MSC scale-up. Microcarriers are not required for MSC scale-up.
VitroGel® MSC is ready-to-use with an optimized formulation that fully supports the rapid expansion of MSCs. Cells directly thawed from liquid nitrogen or passaged from 2D culture vessels can be immediately mixed with the hydrogel solution for 3D culture or hydrogel-cell bead generation. This hydrogel system is compatible with most MSC culture media and tissue culture vessels. By using the VitroGel® Organoid Recovery Solution, cell harvesting after 3D culture is simple and efficient.
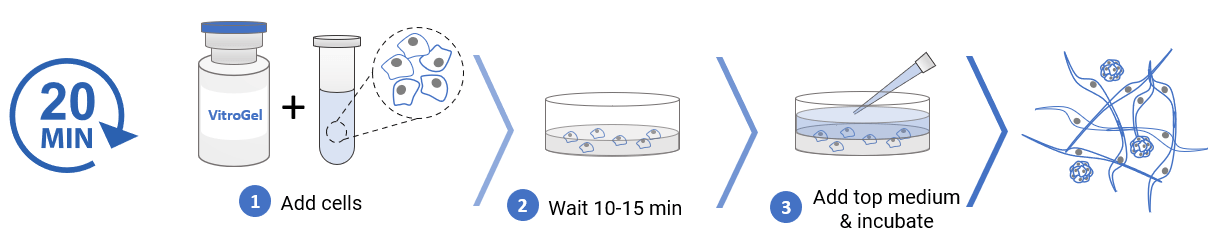
3D cell culture process in 20 min – “Just add cells”
VitroGel® MSC is ready-to-use. Just mix with your cells.
Specifications
| Formulation | Xeno-free, functional hydrogel |
| Use | 3D cell culture, 2D hydrogel coating, hydrogel-cell bead formation |
| Operation | Ready-to-use at room temperature |
| Biocompatibility | Biocompatible, safe for animal studies |
| Injection | Contact support@thewellbio.com for more information. |
| Cell Harvesting | VitroGel Organoid Recovery Solution 5-15 min cell recovery |
| pH | Neutral |
| Storage | Store at 2-8°C. Ships at ambient temperature |
| Sizes | 10 mL and 2 mL |
| Number of Uses | (10 mL) 300 uses at 50 µL per well (2 mL) 60 uses at 50 µL per well |
Recommended Product
Recover cells from the hydrogel within 15 minutes with high cell viability. Non-enzymatic formulation.
Video Protocols & Demonstrations
Research Highlights
Data and References
![]()
MSC 3D Cell Culture
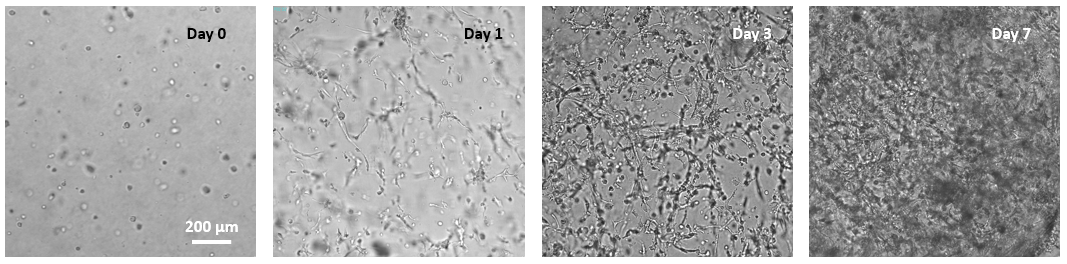
Figure 1. 3D culture of MSC in VitroGel® MSC.
MSC cells were suspended in cell medium at 8 x 105 cells/mL and mixed with VitroGel® MSC for 3D culture (according to the 3D cell culture protocols of VHM03). The images show the growth and expansion of cells inside of 3D hydrogel from day 0 to day 7.
![]()
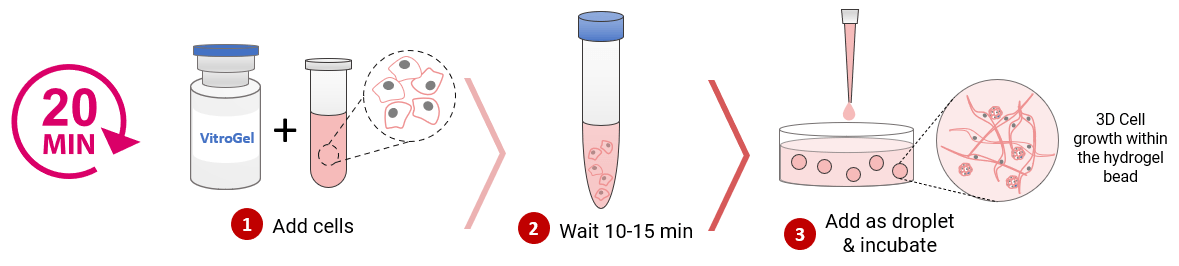
MSC 3D Scale-Up Hydrogel Bead Method
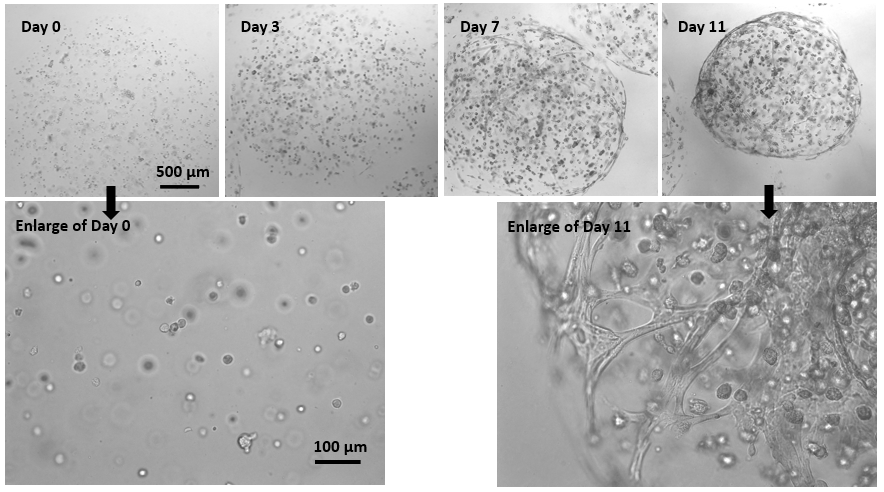
Figure 2. 3D culture of MSC in hydrogel beads.
MSCs were mixed with VitroGel® MSC and added to the cell culture medium as droplets for hydrogel-cell bead formation (according to the hydrogel-cell bead protocols of VHM03). The size of the hydrogel beads can be controlled by the volume of the droplets. MSC cells can grow within the hydrogel beads for long-term culture (>3 weeks). The images show the growth and expansion of cells inside of hydrogel beads from day 0 to day 11. The enlarged images show the cells grew from single cells to cell spheroid and matrix structure from day 0 to day 11.
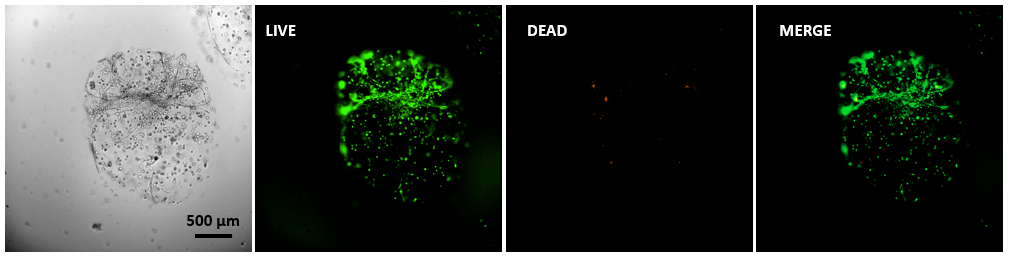
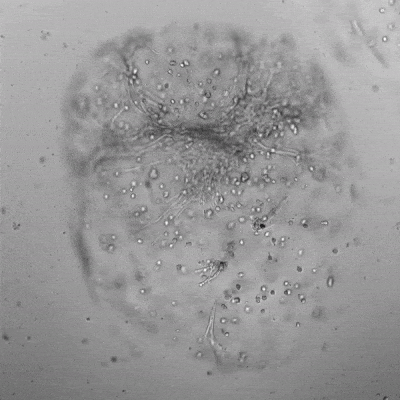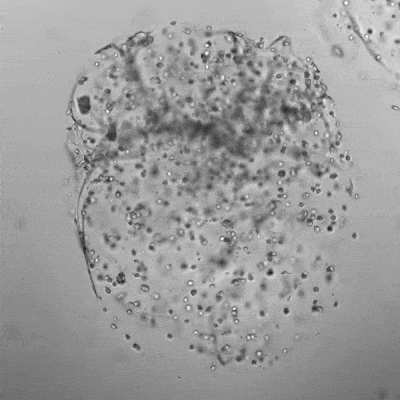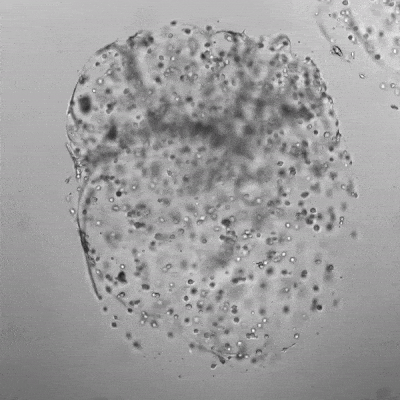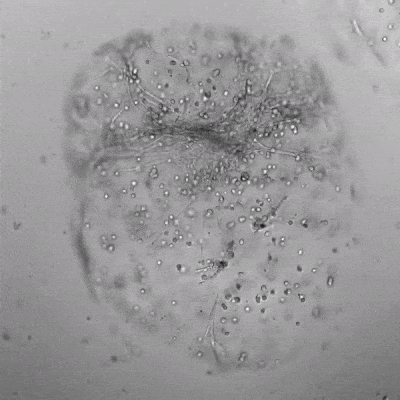
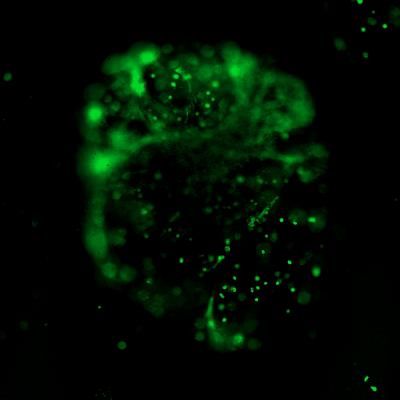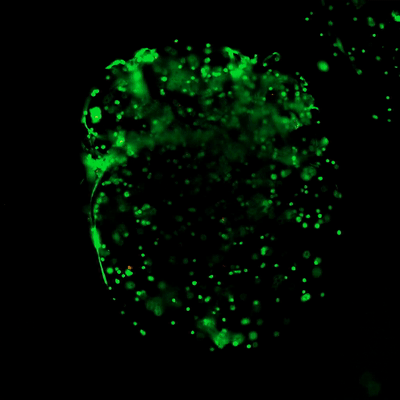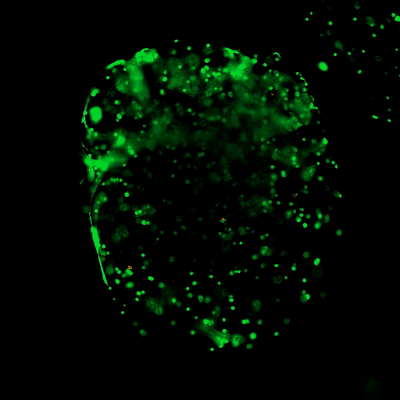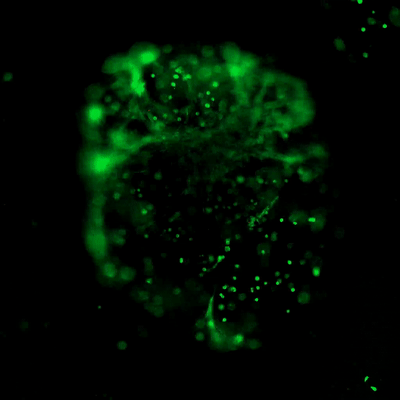
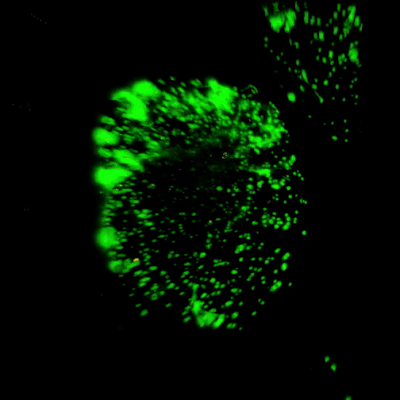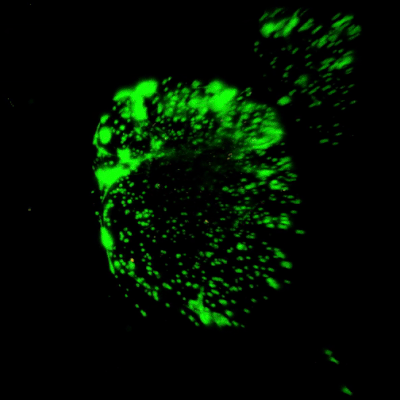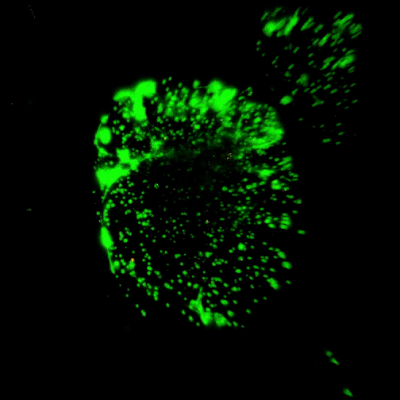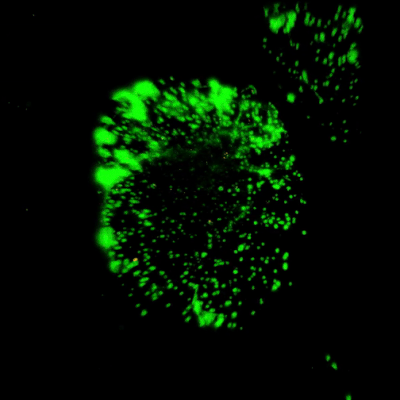
Figure 3. Live/dead assay of MSCs cultured in VitroGel® MSC hydrogel beads.
MSCs cultured in hydrogel beads for 11 days were stained with Cyto3D® Live/Dead Assay Kit (Cat# BM01). The live cells are shown in green color and dead cells are shown in orange color. The images show cells cultured in VitroGel® MSC hydrogel beads perform with high cell viability. The videos present the z-stack images of transmitted light (TL) and live/dead fluorescent images, and the 3D reconstructed image of the hydrogel-MSC bead.
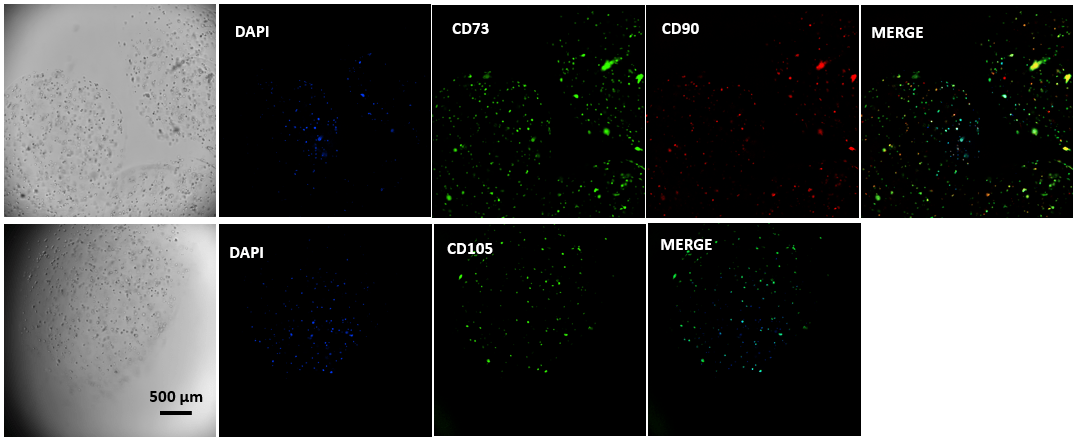
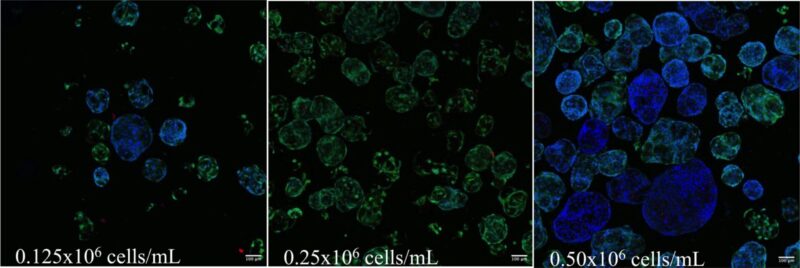
Figure 4. Immunofluorescent images of MSCs cultured in VitroGel® MSC hydrogel beads.
MSCs cultured in hydrogel beads were collected by centrifuging and stained with MSC biomarkers CD73, CD90, and CD105. The images show cells cultured in VitroGel® MSC hydrogel beads presenting all three biomarkers, which indicate the full characteristics of mesenchymal stem cells in 3D hydrogel-MSC beads.
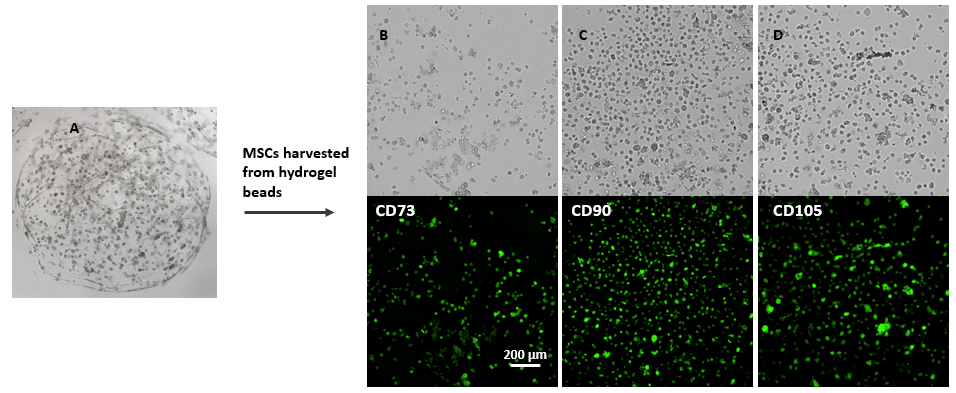
Figure 5. Immunofluorescent images of MSCs after harvesting from VitroGel® MSC hydrogel beads.
MSCs cultured in hydrogel beads for 8 days (A) and then harvested from the hydrogel by using VitroGel® Cell Recovery Solution (check cell recovery protocol for details). Cells were then collected by centrifuging, seeded to glass-bottom tissue culture plate and stained with MSC biomarker CD73, CD90 and CD105. The images show that cells recovered from VitroGel® MSC hydrogel beads present all three biomarkers, which indicate the full characters of mesenchymal stem cells in 3D hydrogel-MSC beads.
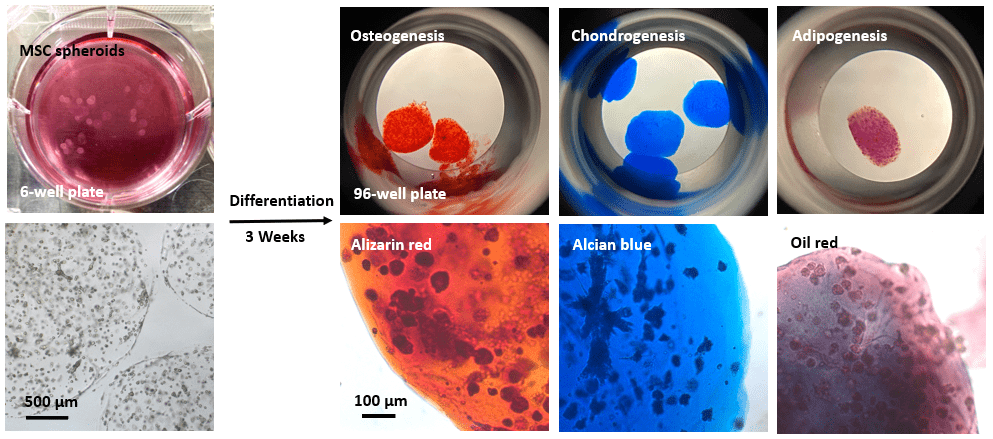
Figure 6. Differentiation of MSCs cultured in VitroGel® MSC hydrogel beads.
MSCs were cultured in hydrogel beads for 7 days. The hydrogel-MSC beads were collected by centrifuging and resuspended in osteogenesis, chondrogenesis and adipogenesis differentiation media, respectively. Cells were differentiated for three weeks. The differentiated cells were collected and stained with alizarin red, alcian blue and oil red, respectively. The images show cells cultured in VitroGel® MSC hydrogel beads can be successfully differentiated into osteocytes, chondrocytes, and adipocytes.
References/Publications
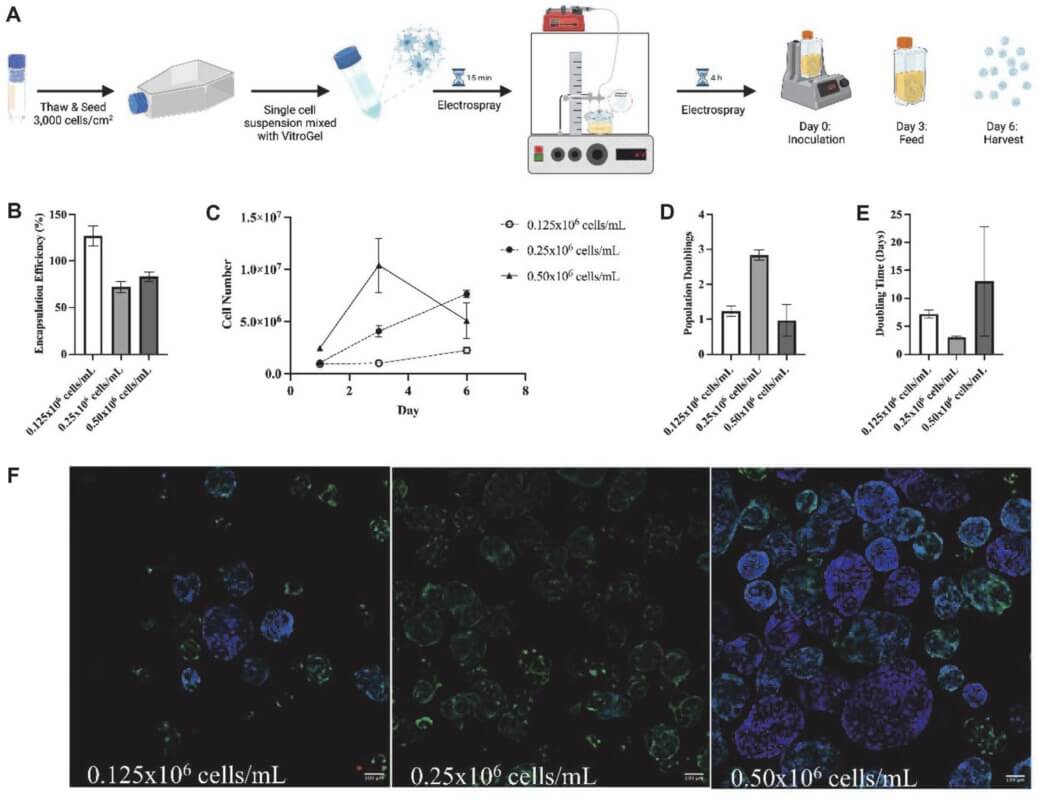
- Teryek, M., Jadhav, P., Bento, R., & Biju Parekkadan. (2024). 3D microcapsules for human bone marrow-derived mesenchymal stem cell biomanufacturing in a vertical-wheel bioreactor. Biotechnology and Bioprocess Engineering. https://doi.org/10.1007/s12257-024-00069-7
- Lee, H. J., Lau, L. N., Sidhu, S. K., Park, J.-Y., & Yeo, I.-S. L. (2025). Three-Dimensional Hydrogel Culture Reveals Novel Differentiation Potential of Human Bone Marrow-Derived Stem Cells. Prosthesis, 7(3), 52. https://doi.org/10.3390/prosthesis7030052
- Teryek, M., Jadhav, P., Bento, R., & Biju Parekkadan. (2023). High-Throughput Production of Microcapsules for Human Bone Marrow Derived Mesenchymal Stem Cell Biomanufacturing in a Vertical-Wheel Bioreactor. Biotechnology and Bioprocess Engineering, 28(4), 528–544. https://doi.org/10.1007/s12257-023-0020-9
- Powell K. Adding depth to cell culture. Science, 356(6333), 96–98. https://doi.org/10.1126/science.356.6333.96
| Size | 2 mL, 10 mL |
|---|