Research Highlights
Decoding the Crucial Role of Carotid Body in Maximizing Exercise Metabolism with VitroGel
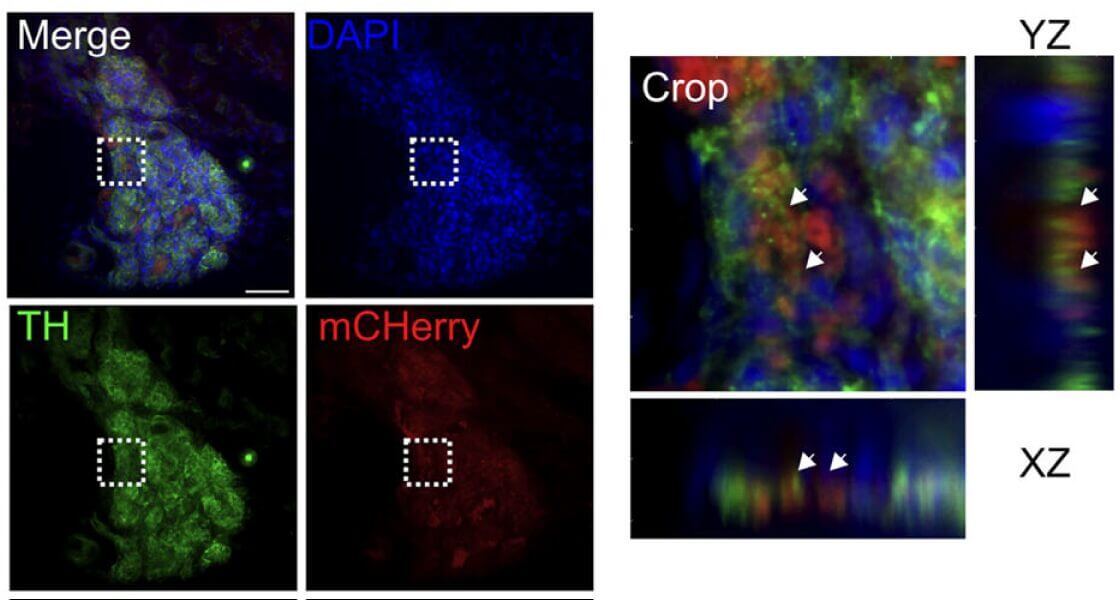
VitroGel® 3D enables precise chemogenetic targeting of carotid body cells to uncover metabolic function.
Category:
Injectable / In vivo
Cell Types:
Adeno-associated viruses (AAVs)
Hydrogel:
VitroGel® 3D (TWG001)
Institutions:
Exercise Applied Physiology Laboratory, Faculty of Health Sciences, University of Antofagasta
Team:
David C. Andrade, Camila Salazar-Ardiles, Camilo Toledo, Cristian Alvarez, Esteban Díaz-Jara, Maria Rodriguez-Fernandez, Gregoire P. Millet, Rodrigo Iturriaga
During intense exercise, our bodies have a “ceiling” for oxygen consumption, known as peak oxygen uptake (VO₂ peak), which is a key determinant of athletic endurance. For decades, scientists have known that a tiny organ in the neck, the carotid body, acts as a master sensor for blood oxygen levels. Recently, it has been proposed that it also senses metabolic byproducts, such as lactate, potentially playing a crucial role in pushing our oxygen uptake to its limits during maximal exertion. Traditional methods like surgical removal are irreversible and invasive, while injecting genetic tools in a liquid solution is imprecise and risks the material washing away, failing to target the minute carotid body tissue effectively.
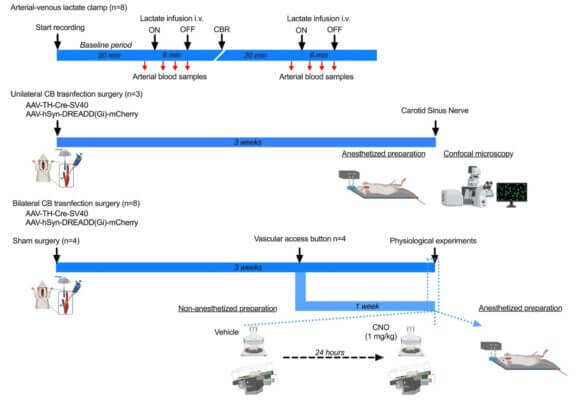
In this study, the research team used an innovative chemogenetic technique. They designed a virus to deliver a “silencing switch” (a DREADD receptor) exclusively into the glomus cells of the carotid body in rats. The critical step was the local delivery of this virus. They used VitroGel® 3D, mixing it with the viral solution to create a stable and biocompatible gel-virus mixture. This gel-virus mixture was then carefully applied directly to the carotid bodies. The hydrogel acted as a protective reservoir, ensuring the viruses remained at the injection site long enough to infect the target glomus cells efficiently. This method was far superior to liquid injection, guaranteeing high, localized expression of the silencing receptor. Once the switch was in place, administering a designer drug (CNO) specifically inhibited the carotid body cells, allowing the team to observe the physiological consequences during exercise tests.
By using VitroGel® to enable precise chemogenetic inhibition, the study provided the first direct evidence that the carotid body is indeed a metabolic sensor essential for achieving peak oxygen uptake. When the carotid body was silenced, the VO₂ peak decreased significantly, demonstrating its critical role in maximizing oxygen use during exercise. The VitroGel® system was instrumental in this discovery by solving the critical challenge of targeted delivery to a small, delicate structure. This success demonstrates how advanced hydrogel biomaterials like VitroGel® are vital tools for cutting-edge physiological research.
PRODUCT USED:
Discover how VitroGel® can support your cell culture research
Explore our updated resources for a concise overview of VitroGel®’s key features and application areas.
Comparison of VitroGel® vs. Animal-based ECM
Discover the 20+ advantages of VitroGel® over animal-based ECM with this comprehensive comparison on key features, operation, application, and storage conditions.
Learn MoreWhat is VitroGel® | Overview Video
Learn why VitroGel® is the leading animal-free hydrogel for 3D cell culture.
Learn MoreVitroGel® for Xenograft
Learn how VitroGel® provides well-defined and full control of the microenvironment with consistent results.
Learn More