Conferences, News
American Association for Cancer Research (AACR) – 2025

The future of cancer research is here—and you’re invited to be part of it! Join us at AACR 2025 (American Association for Cancer Research) from April 25-30, 2025, at McCormick Place Convention Center, Chicago, IL, and explore the latest advancements in cancer research, discover innovative solutions, and connect with our team of experts.
AACR 2025 – Booth #544
Stop by booth #544 to get insights on our new product launches or connect with our team of experts.

Presenting with VitroGel®? Notify us to receive a special gift!
If you will be presenting research utilizing VitroGel® hydrogels in either a poster or oral presentation at an upcoming scientific conference, we invite you to notify us of your participation.
Submit your details to qualify:
Submit your PresentationPoster Sessions
Click to expand to view more details on the poster session presented by collaborators/users of VitroGel®.
https://www.abstractsonline.com/pp8/#!/20273/presentation/4956
Date: Sunday, April 27, 2025
Time: 2:00 PM – 5:00 PM
Session Category: Tumor Biology
Location: Poster Section 6
Poster Board Number: 7
Abstract #136 | Discerning the Role of the Extracellular Matrix on Epithelial-to-Mesenchymal Transition and Invasiveness of Glioblastoma Multiforme using a Xeno-Free 3D Hydrogel System
The invasive nature of cancer, driven by epithelial-to-mesenchymal transition (EMT), is a critical process influenced by external cues from the tumor microenvironment, such as extracellular matrix (ECM) composition. The ECM primes cells for EMT and metastasis, making it a key topic in understanding disease progression. For example, in glioblastoma multiforme (GBM), a grade IV astrocytoma and the most aggressive brain malignancy, EMT contributes to the highly invasive behavior of tumor cells, limiting patient survival to less than five years. Thus, developing in vitro models that can accurately examine how the ECM contributes to GBM invasiveness and survival is vital. Currently, most in vitro studies evaluating cell invasion and EMT are conducted in two-dimensions (2D), disregarding the microenvironment’s complexity and the ECM role in such processes. The limited case studies on three-dimensional (3D) cancer invasion and EMT models heavily rely on animal-based ECM, such as Matrigel or collagen. These matrices, due to their inherent instability, introduce variability, compromise experimental consistency, and make it challenging to draw robust conclusions. In this study, we use the synthetic and biofunctional VitroGel® system to evaluate cell invasion and EMT in 3D GBM models. The fully characterized VitroGel system allow us to study the effect of the mechanical strength and biochemical composition of the ECM on cell invasion and EMT. In this study, GBM spheroids were formed in an ultra-low attachment plate, and hydrogels functionalized with ligands such as RGD peptides, matrix metalloproteinases, collagen-mimetic peptides, and others were gently added to the cultures to evaluate their role in cell invasion and EMT. The findings showed that the functional ligands stimulate spheroid invasion and promote EMT, evidenced by the rapid proliferation of the spheroid and engulfment of the matrix, resulting in the formation of tumoroid-like structures. Furthermore, we evaluated whether the mechanical strength of the ECM affects spheroid invasion and EMT by adjusting the stiffness of the matrix. Indeed, we showed that less stiff matrices propel spheroid invasion and EMT, indicating that the physical properties of the ECM influence migration. Overall, this study demonstrated that using the xeno-free 3D hydrogel system is an excellent approach to evaluate cancer invasiveness and EMT, and a promising tool for drug-screening applications.
https://www.abstractsonline.com/pp8/#!/20273/presentation/4075
Date: Sunday, April 27, 2025
Time: 2:00 PM – 5:00 PM
Session Category: Tumor Biology
Location: Poster Section 4
Poster Board Number: 2
Abstract #0079 | Pax6 regulates stemness and enhances brain metastases in breast cancer
Author: Laiba Anwar
Institution: University of Nebraska Medical Center, Omaha, NE
Brain metastasis (BrM) remains the most severe complication of breast cancer (BC), the most diagnosed cancer in females, associated with an abysmal prognosis. Our poor understanding of the molecular mechanisms driving the disease is one of the major reasons for the dearth of specific therapies. This highlights an urgent clinical need to decipher how BC cells form BrM. BCBrM is preceded by a period of dormancy, during which stem cell-like features of cancer cells may play a role in reactivating them to form metastatic lesions. Cancer stem cells, known as “brain tumor-initiating cells” contribute to tumor formation in the brain and could also lead to radio and chemoresistance in the treatment of brain metastases. We, therefore, conducted a bioinformatic analysis on publicly available transcriptomic datasets of matched primary and brain metastatic patient samples and found that Pax6, a transcription factor, was upregulated in the metastatic samples compared to the primary tumors. In tissue microarray (TMA) belonging to BrM patients, Pax6 expression was evident in the BrM lesions and nearly absent in normal brain tissue. Pax6 was found to be upregulated in BCBrM cell lines compared to primary BC cell lines. Pax6, a master regulator of transcription, essential for normal development of the eyes and central nervous system, has been implicated in cancer progression. Pax6 can promote proliferation, migration, and resistance to apoptosis. To delineate the essentiality of Pax6 in BCBrM, we performed CRISPR/Cas9-mediated Pax6 knockout (KO) in the BCBrM cell lines, which decreased proliferation, wound healing, and migration properties. Intracardiac injection of these Pax6 KO BCBrM cells into immunocompromised mice resulted in a significant reduction of brain metastatic tumor lesions compared to controls. Conversely, overexpression of Pax6 in primary BC cells induced a BrM-like phenotype, enhancing proliferation, migration, and wound healing. Since Pax6 regulates stem cell features we also found that Pax6 KO reduced the expression of stemness-promoting factors such as OCT4, NANOG, and KLF4. Further, to confirm the stemness features we found that in serum-free conditions, Pax6 KO cells significantly lacked mammosphere-forming abilities and side populations. These findings suggest that Pax6 mediates stemness in cancer cells and enhances the metastatic potential of BCBrM cells. Unraveling the role of Pax6 in BCBrM could potentially open new therapeutic avenues for the treatment of BCBrM patients which eventually will improve outcomes of these patients.
https://www.abstractsonline.com/pp8/#!/20273/presentation/8705
Date: Wednesday, April 30, 2025
Time: 9:00 AM – 12:00 PM
Session Category: Tumor Biology
Location: Poster Section 3
Poster Board Number: 8
Abstract #6497 | Modeling HCC molecular and histological features by CRISPR editing of primary hepatocytes
Authors:
Lobna Elkhadragy, Olayinka G. David, Caitlyn C. Castillo, Isadora Andre Rosa Lopes, Hannah Liu, Luke R. Jordan, Luke N. Redlon1, Grace Guzman, Jonathan P. Samuelson, Lawrence B. Schook1, Amaia Lujambio, Kyle M. Schachtschneider, Ron C. Gaba
Institutions:
- Department of Radiology, University of Illinois at Chicago, Chicago, Illinois, USA
- Department of Pathology, University of Illinois at Chicago, Chicago, Illinois, USA
- Department of Veterinary Clinical Medicine, University of Illinois at Urbana-Champaign, Champaign, Illinois, USA
- Department of Oncological Sciences, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Background:
Hepatocellular carcinoma (HCC) is an aggressive disease with poor response to current therapies, highlighting the need for more effective therapeutic strategies. Preclinical HCC models are necessary tools for assessment of novel therapies. Pigs offer a valuable disease model due to their similarities with humans and their large size which allows testing clinically relevant procedures. Here, we aimed to develop HCC cell and animal models by precise gene manipulation of primary porcine hepatocytes.
Materials and Methods:
Hepatocytes were isolated from minipigs (n=4) and subjected to CRISPR knockout (KO) of tumor suppressor genes, with or without oncogene overexpression (OE). Gene alteration combinations included KO of TP53, PTEN, and/or CDKN2A, with or without c-myc overexpression. Gene alterations were validated by Sanger sequencing, PCR, and Western blotting. The developed cell lines were injected subcutaneously into severe combined immunodeficient (SCID) mice to confirm their tumorigenicity and assess histological features. Cells with TP53KO; PTENKO; CDKN2AKO; c-mycOE (TPKM) were then autologously injected into pigs (n=2) by percutaneous ultrasound-guided liver parenchymal injection and tumor development was monitored by ultrasound and CT imaging. Tumors from mice and pigs were analyzed by histological and transcriptional analyses.
Results:
The induced gene alterations in porcine hepatocytes resulted in the successful development and propagation of four distinct cell lines, all validated for the intended modifications. Injection of these cells into SCID mice resulted in tumor development confirming in vivo tumorigenicity. Histological analysis of mouse xenograft tumors identified epithelial neoplasms with multiphenotypic cells, displaying moderate to poor differentiation, and closely mimicking human liver cancer. In pigs, autologous injection of TPKM cells resulted in intrahepatic mass formation within 3 weeks. Histological analysis of the porcine masses revealed neoplastic cells with positive Arginase staining, negative CK19 staining, and intratumoral immune cell infiltration. Transcriptional analysis of the mouse and pig tumors showed activation of pathways associated with cancer cell proliferation and hepatic fibrosis.
Conclusion:
This study successfully establishes genetically defined HCC models with histological and molecular features resembling human HCC by CRISPR editing of primary porcine hepatocytes. These innovative HCC cell models provide valuable tools for drug screening and mechanistic studies, and the porcine model is a promising tool that harbors defined tumor driver mutations and is suitable for locoregional therapy testing. Together, these novel HCC cell and animal models offer promising avenues for preclinical evaluation of innovative therapeutic strategies.
https://www.abstractsonline.com/pp8/#!/20273/presentation/9904
Date: Wednesday, April 30, 2025
Time: 9:00 AM – 12:00 PM
Session Category: Tumor Biology
Location: Poster Section 3
Poster Board Number: 8
Abstract #LB472 | Role of PLCG2 in colorectal cancer development and progression
Authors:
Onur M. Batmaz, Xiaodong Wang, Vera Leidig, Dominik Saul, Robyn Laura Kosinsky
Institution: Robert Bosch Center for Tumor Diseases, Stuttgart, Germany
Colorectal cancer (CRC) has the fourth highest incident with the third highest mortality rate among all cancer types with global five-year survival rate of 65%. Although, due to advances in non-invasive imaging technologies, CRC can be detected at early stages, the number of incidents increase yearly. Therefore, uncovering new mechanism underlying CRC development and progression is crucial to provide improvement in making diagnostic and prognostic outcome assessment in the clinical field. For these reasons, we determined CRC tumor cell expression profiles in several publicly available scRNA-seq data sets. Interestingly, we found that although PLCG2 expression largely downregulated in most epithelial cells, some cells retained their expression. These PLCG2-High (PLCG2-H) cells found to be enriched in their proliferative and metastatic capabilities compared to PLCG2-Low (PLCG2-L) tumor epithelial cells. We tested our observations from scRNA-seq datasets in vitro by inducing suppression and over-expression of PLCG2 in human CRC cell lines. Our functional assays as well as RNA-seq analyses have shown that suppression of PLCG2 decreased migratory capacity of CRC could enable CRC cells to become more metastatic, and have the potential to be used as a biomarker to make improved prognostic outcome assessment for CRC patients. These findings suggest that retained PLCG2 levels could enable CRC cells to become more metastatic, and have the potential to be used as a biomarker to make improved prognostic outcome assessment for CRC patients.
Don’t miss out!
- NEW Product Developments
Get a sneak peek of our latest innovations and help elevate your cell culture research. - Win Exciting Prizes!
Score a chance to win exclusive giveaways and get a chance to play in our TheWell of Fortune.
- Talk to our experts!
Learn how the xeno-free VitroGel® hydrogels can replace animal-derived ECM for your 3D cell culture research. - Meet the latest addition to our team – Alexis TheWhale!

New products we will be showcasing:
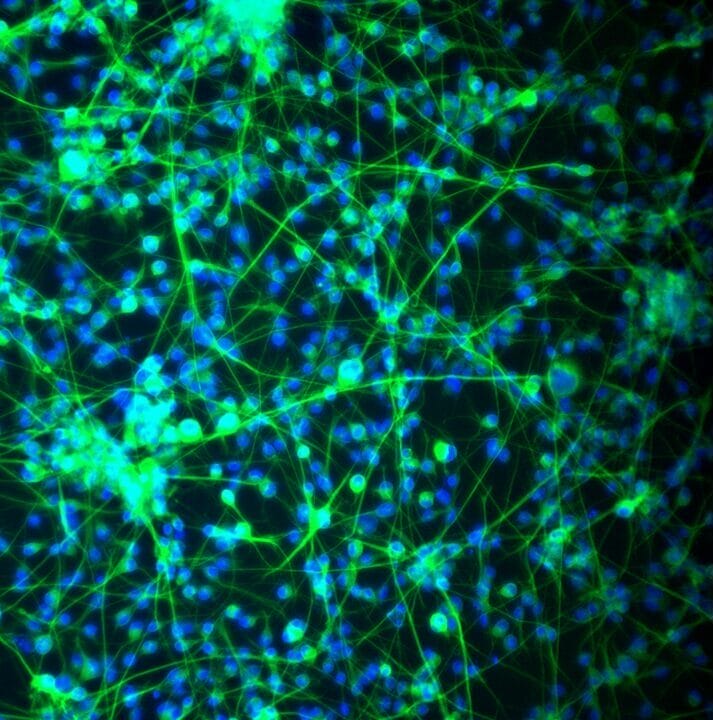
VitroGel® NEURON
A synthetic matrix with functional ligands that support the maintenance and differentiation of iPSC-derived neural stem cells, cultured as single cells or spheres, and culture of mature neurons.
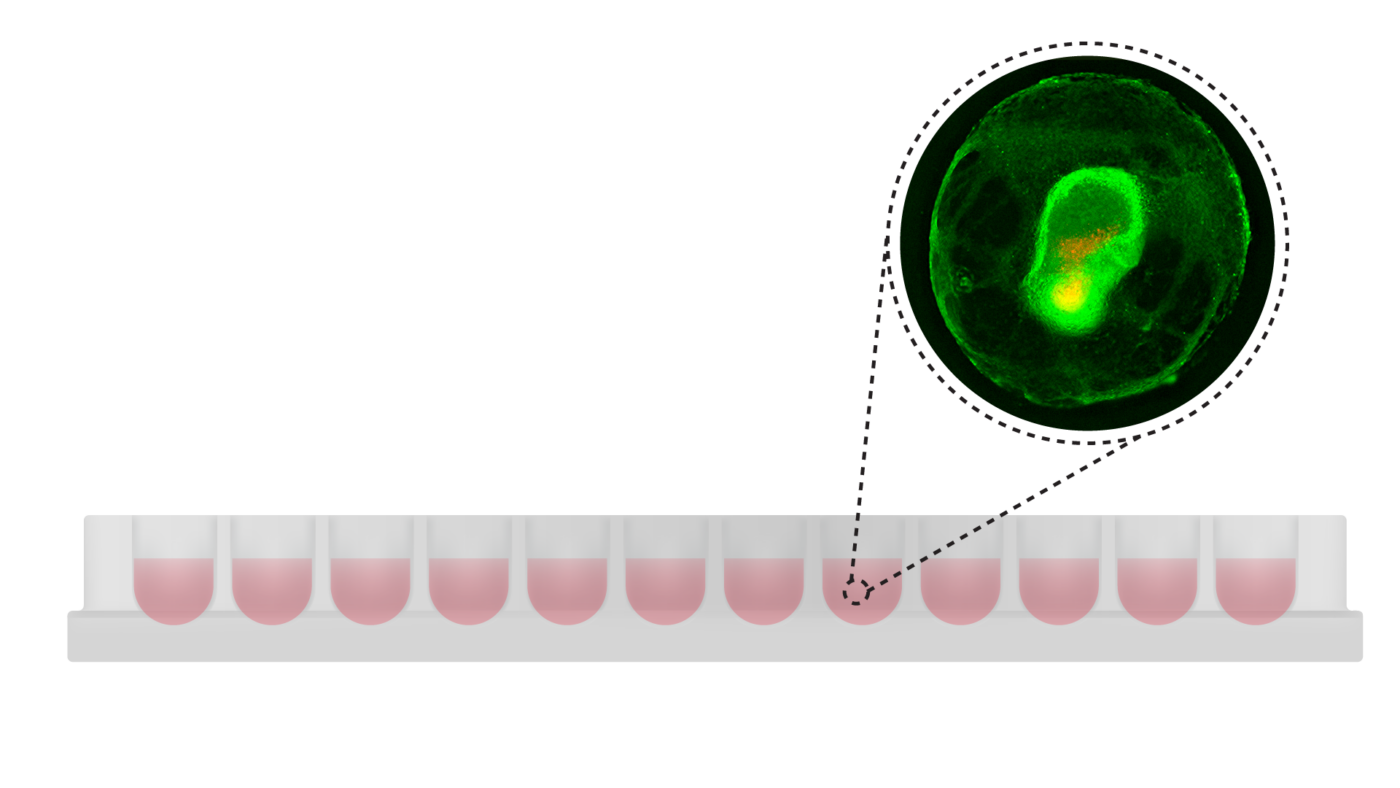
VitroPrime™ Ultra-Low Attachment Plate, U-Bottom
Premium plate for 3D spheroid formation.
VitroPrime™ Ultra-Low Attachment Plate, 96-well, U-bottom is an ultra-low attachment plate which inhibits cell attachment ideal for 3D spheroid and tumoroids/organoid culture.

CytoGrow™ Growth Factors and Supplements
Premium quality growth factors and small molecules designed to support consistent, high-performance cell culture.
Meet Up with our Experts!
We would love to meet you. Want to schedule a time with our team at AACR or can not attend? Please fill the form and our team will be happy to assist you in any 3D cell culture applications with our VitroGel® hydrogels.

3D Organoid Culture
Kalhara Menidiwela, Ph.D.
Research Scientist II

3D Cell Culture
Alejandra Ferrer Díaz, Ph.D.
Research Scientist II