Application Notes
Evaluating Spheroid Invasion with VitroGel® Hydrogel Matrix in VitroPrime™ Ultra-Low Attachment, U-Bottom, 96-well Plate
Application Note
Alejandra Ferrer Díaz, Ph.D., John Huang Ph.D.
TheWell Bioscience Inc., Monmouth Junction, NJ

Introduction
Spheroid invasion assays offer a physiologically relevant three-dimensional (3D) in vitro model for studying cancer cell behavior, surpassing the limitations of traditional two-dimensional (2D) cultures [1]. Spheroids are compact cell aggregates that recapitulate critical in vivo features, including cell–cell and cell–matrix interactions, as well as nutrient and oxygen gradients providing a more accurate representation of a solid tumor biology [2]. As a result, spheroid models are especially valuable for investigating invasion dynamics, metastatic potential, and therapeutic responses within a controlled extracellular matrix environment [2, 3]. In this study, we employed a spheroid-based invasion assay to evaluate glioblastoma (GBM) cell invasion in a 3D context. Spheroids were formed using the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate and subsequently embedded in VitroGel® Hydrogel Matrix to monitor radial cell invasion from the spheroid core into the surrounding matrix.
Materials
- Hydrogel: VitroGel® Hydrogel Matrix (Cat. #: VHM01)
- Plate: VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well plate (Cat. #: VP-ULA96U-8)
- Cells: U87-MG glioblastoma cells
- Complete Medium: Minimal Essential Medium (MEM) with 10% FBS
Methods
Cell Culture
U87-MG glioblastoma cells were cultured in MEM with 10% FBS supplemented with 1X glutamax and penicillin-streptomycin. The cultures were passaged at 80-90% confluency.
Spheroid Formation
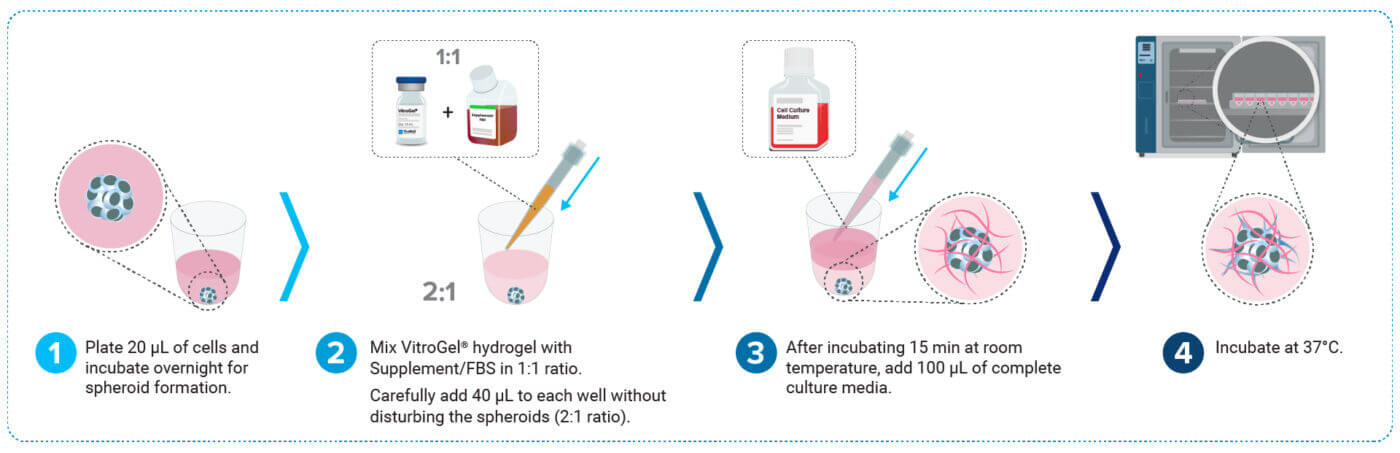
U87-MG glioblastoma cells were harvested and resuspended at a concentration of 1 × 10⁶ cells/mL in complete culture medium. A volume of 20 μL of this suspension was added to each well of the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well plate to promote single spheroid formation per well. Plates were incubated overnight at 37°C (Figure 1, step 1).
Hydrogel Embedding and Invasion Assay
VitroGel® Hydrogel Matrix, FBS, and culture medium were equilibrated to room temperature. A 1:1 hydrogel-FBS mixture was gently homogenized and added to the wells containing spheroids in a 2:1 hydrogel-to-medium ratio (e.g., 40 μL of hydrogel was added to 20 μL of spheroid-containing medium). To preserve spheroid integrity, the hydrogel was dispensed against the wall of the well while tilting the plate. After a 15-minute room-temperature incubation to stabilize the hydrogel, 100 μL of complete medium was gently added on top of the hydrogel (Figure 1, steps 2-3). Spheroids were incubated at 37°C and monitored daily to assess radial invasion into the hydrogel matrix using brightfield microscopy (Figure 1, step 4). Thirty percent of the medium in culture was replaced every 2-3 days with fresh medium.
Results and Discussion
Spheroid Formation
Spheroid formation is a crucial step in the invasion assay workflow that relies heavily on the quality of the plasticware used [4]. The spheroid invasion assay specifically requires ultra-low attachment (ULA), U-bottom, 96-well plates to promote cell aggregation, causing the formation of spheroids. A significant issue with many ULA, U-bottom plates is the inconsistent formation of spheres across the wells, which compromises experimental reproducibility and hampers laboratory automation capabilities, as well as drug screening applications. In this study, we utilized the premium-quality VitroPrime™ Ultra-low Attachment, U-bottom, 96-well Plate to promote spheroid formation in glioblastoma cells. To induce spheroid formation, U87-MG glioblastoma cells were seeded into the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate and commercially available ULA premium and standard plates. The cells were incubated overnight at 37°C to facilitate spheroid formation. As illustrated in Figure 2 (top), the glioblastoma cells in the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate formed a single spheroid, with no residual cells observed on the edges of the plate. However, we observed that the cells in the commercially available plates failed to form round-shaped spheroids, which is crucial when performing spheroid invasion assays (Figure 2, middle and bottom sections). These findings indicate that the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate is highly effective for consistent spheroid formation.
Comparison of spheroid formation between VitroPrime™ Ultra-Low Attachment, U-Bottom, 96-well Plate and 2 commercially available ultra-low attachment plates
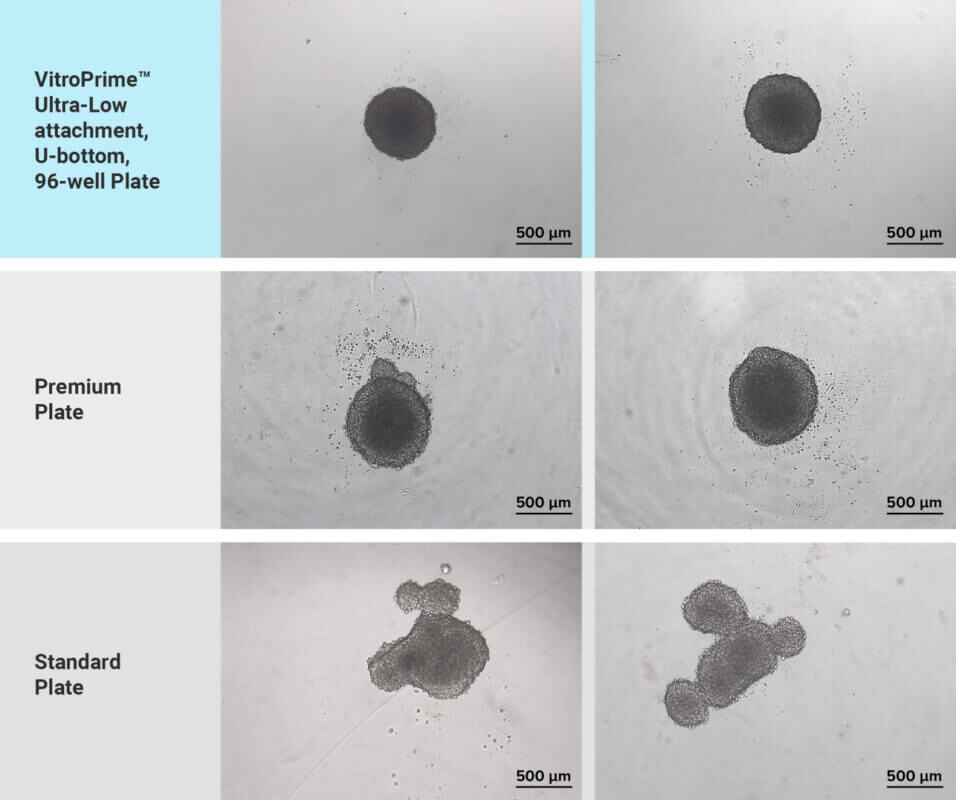
Figure 2: Spheroid formation evaluation in VitroPrime™ Ultra-Low Attachment, U-Bottom, 96-well Plate and commercially available plates. U87-MG glioblastoma cells were resuspended in basal medium with 10% fetal bovine serum. Twenty microliters (20 μL) of cell suspension were added to the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate, and commercially available ultra-low attachment plates. The cultures were incubated overnight at 37°C to allow spheroid formation. Brightfield images were obtained using a Zeiss microscope at a 4X magnification.
Spheroid Invasion Assay with VitroGel® System
A key factor in evaluating spheroid invasion in vitro is the use of an extracellular matrix (ECM). While most invasion studies utilize animal-based ECMs such as Matrigel or Geltrex, these matrices polymerize based on temperature and lack proper characterization, negatively impacting experimental outcomes. Herein, we used the synthetic, xeno-free VitroGel® Hydrogel Matrix to examine glioblastoma spheroid invasion. This pure synthetic hydrogel was functionalized to closely mimic the natural extracellular matrix (ECM) and promote cell-matrix and cell-cell interactions. The hydrogel is in a liquid form at room temperature, making the spheroid invasion assay setup easy and straightforward. To perform the spheroid invasion assay, we combined the hydrogel with FBS in a 1:1 ratio, added 40 μL of the mixture to the wells with spheroids, followed by a 15-minute incubation at room temperature. Then, 100 μL of complete medium was added on top of the hydrogel, and the cultures were placed in the incubator set at 37°C. The cultures were monitored using an inverted microscope, and images were captured on days 3, 6, 11, 15, 22, 30, 35, and 41.
During the early stages of spheroid invasion, we observed that the spheroids increased in size, with only a few cells beginning to protrude from the main body (Figure 3, days 3 and 6). However, by days 11, 15, and 22, invasion became more pronounced, as the invasive cells continued to degrade the hydrogel matrix (Figure 3). By the later time points, both spheroid growth and invasion throughout the matrix had significantly increased (Figure 3, days 30, 35, and 41).
The combination of the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate and the xeno-free VitroGel® Hydrogel Matrix is excellent for spheroid invasion studies resulting in consistent assay results and simple experiment setup with lab automation capability. The 3D spheroid assay setup provides a versatile platform to evaluate cell invasion dynamics from a structured tumor mass into a tunable matrix environment.
Spheroid Invasion Assay
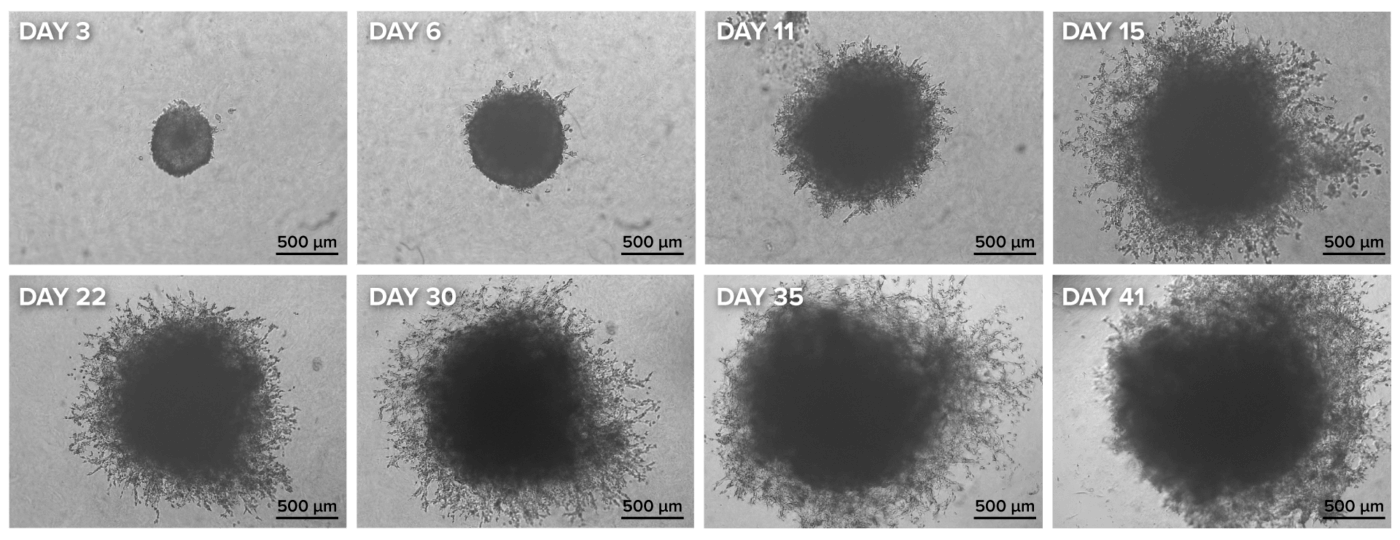
Figure 3: Spheroid invasion assay using the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate and VitroGel® Hydrogel Matrix. U87-MG glioblastoma spheroids were generated using the VitroPrime™ Ultra-Low Attachment, U-bottom, 96-well Plate as described in Figure 2. VitroGel® Hydrogel Matrix was combined with serum, and 40 μL of the mixture was added to the spheroid, followed by a 15-minute incubation at room temperature. The cultures were imaged on days 3, 6, 11, 15, 22, 30, 35, and 41 with the Zeiss Microscope at a 2.5X magnification.
References
- Mangani S, Kremmydas S, Karamanos NK. Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers (Basel). 2025, 17. PMID: 40227664.
- Yun C, Kim SH, Kim KM, Yang MH, Byun MR, Kim JH, Kwon D, Pham HTM, Kim HS, Kim JH, Jung YS. Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research. Int J Mol Sci. 2024, 25. PMID: 38473760.
- Guzzeloni V, Veschini L, Pedica F, Ferrero E, Ferrarini M. 3D Models as a Tool to Assess the Anti-Tumor Efficacy of Therapeutic Antibodies: Advantages and Limitations. Antibodies. 2022, 11, 46.
- Xing C, Kemas A, Mickols E, Klein K, Artursson P, Lauschke VM. The choice of ultra‐low attachment plates impacts primary human and primary canine hepatocyte spheroid formation, phenotypes, and function. Biotechnology Journal. 2024, 19.