Blog
Kalhara Menikdiwela Ph.D.
TheWell Bioscience Inc., Monmouth Junction, NJ

Epithelial polarity, the organized distinction between an apical surface (that faces lumen or the outside) and a basolateral surface (that interfaces with stroma and blood) is a fundamental feature of many tissues, especially the intestinal epithelium. In recent years, three-dimensional (3D) organoid models have become central to nutrition, pharmacology, infection biology, and personalized medicine because they recapitulate key aspects of tissue architecture and function. But an often-underappreciated variable in organoid biology is which way the epithelium faces: apical-in (apical side facing an internal lumen) or apical-out (apical side facing the culture medium) (Figure 1). That orientation profoundly shapes what experiments are possible, how readouts must be interpreted, and how faithfully the model represents in-vivo physiology. Below we summarize why polarity matters and give practical implications for researchers drawing on the recent Advances in Nutrition review and other primary literature (1,2,3).
What “Apical-in” and “Apical-out” Mean for Organoid Experiments
Most classic organoid protocols embed cells in basement-membrane-derived hydrogels (e.g., Matrigel). In those contexts, epithelial cells form a polarized epithelium with the apical surface facing an enclosed lumen and the basal surface contacting the matrix in the apical-in configuration (Figure 1). This arrangement faithfully captures many aspects of tissue organization but prevents direct access to the apical surface (e.g., microbes, nutrients, orally administered drugs, or particulate matter). Conversely, apical-out organoids present their apical surface directly to the culture medium, enabling straightforward exposure and manipulation of that functional interface (1,2). The difference is not just geometric; it changes access, transport dynamics, barrier assays, and how one models host–microbe interactions (2).

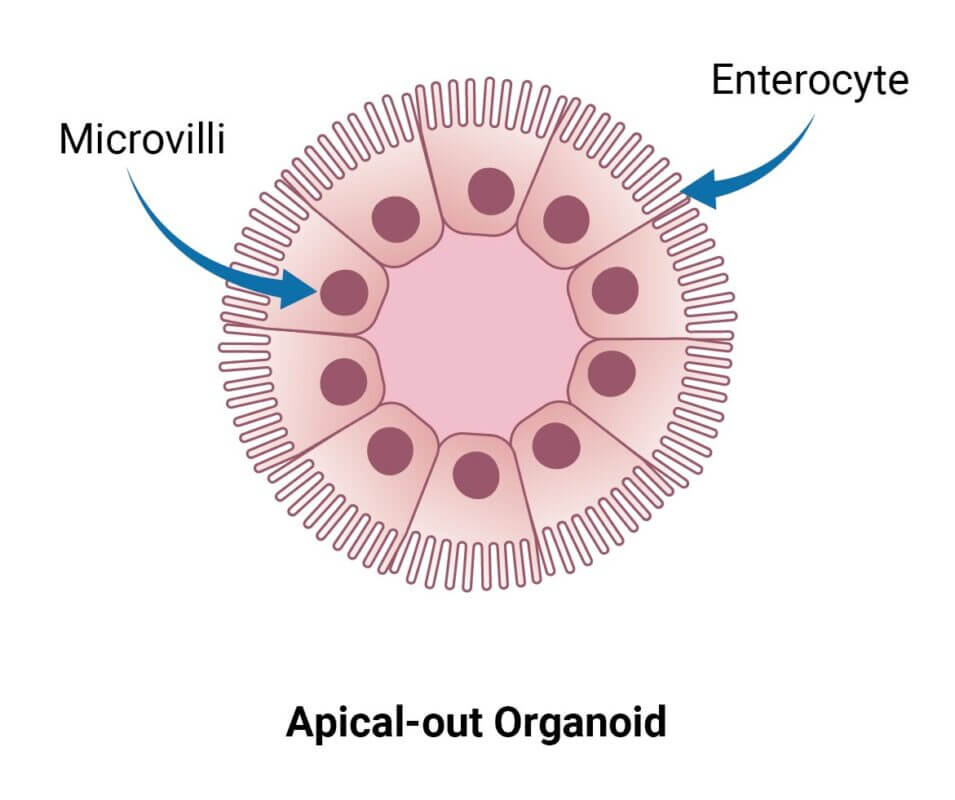
Figure 1. Polarity of Organoids.
A. An organoid with apical-in polarity and epithelial cells facing the center lumen (commonly associated with organoids cultured in animal-based hydrogels. B. An apical-out organoid with epithelial cells facing outside and directly interacting with media and outer environment (naturally supported by VitroGel® ORGANOID 3)
Why Polarity Matters for Nutritional Research
Nutrition research frequently asks how nutrients are taken up, metabolized by the epithelium, or modified by gut microbes before systemic absorption. Apical-out organoids make the apical brush border and its transporters directly accessible, simplifying assays of nutrient uptake, transporter kinetics, and short-term exposure to dietary components or bioactive compounds. Essential functional readouts, e.g., uptake of glucose or lipids, bile acid transformations at the apical membrane, or short-chain fatty acid effects produced by microbes, are easier and more physiologically interpretable when the apical surface is outwardly exposed. The Advances in Nutrition review emphasizes organoids as a bridge between 2D systems and in vivo work in nutrition science; polarity is a central feature that determines which experimental questions organoids can best answer (1).
Drug Discovery, Infection, Microbiome, and Barrier Assays Made Practical by Apical-out Models
Apical-out organoids can be widely used for drug discovery, infectious disease research, and microbiome research (Figure 2). Organoids cultured in animal-based hydrogels with basal out polarity do not support drug absorption at the apical side of epithelial cells, as the epithelial layer is not accessible in basal out organoids. Additionally, pathogens and commensals normally contact the apical epithelium; thus, apical-out orientations allow microbes (or microbial products and metabolites) to access the correct cellular surface without artificial microinjection or disruption of the organoid lumen. This enables infection time courses, live imaging, and controlled co-culture with bacteria or viruses under conditions that preserve epithelial barrier architecture. Several groups have shown that apical-out organoids retain tight junctions, transporter expression, and barrier integrity while allowing robust microbial interactions a critical advantage for modeling diet-microbe-host and drug-host interactions (1,2).
Figure 2. The orientation of epithelial interactions in organoids with different polarities.
A. Nutrients, drugs, and microbes interact with the epithelial layer of the apical side of enterocytes. B. An apical-in organoid interacts with drugs, nutrients, and microbes at the basolateral side of the organoid. C. Apical-out organoids mimic the natural, in vivo direction of the drug, nutrient interaction with epithelial cells facing outside (organoids cultured in VitroGel® ORGANOID 3).
How Researchers Generate Apical-out Organoids
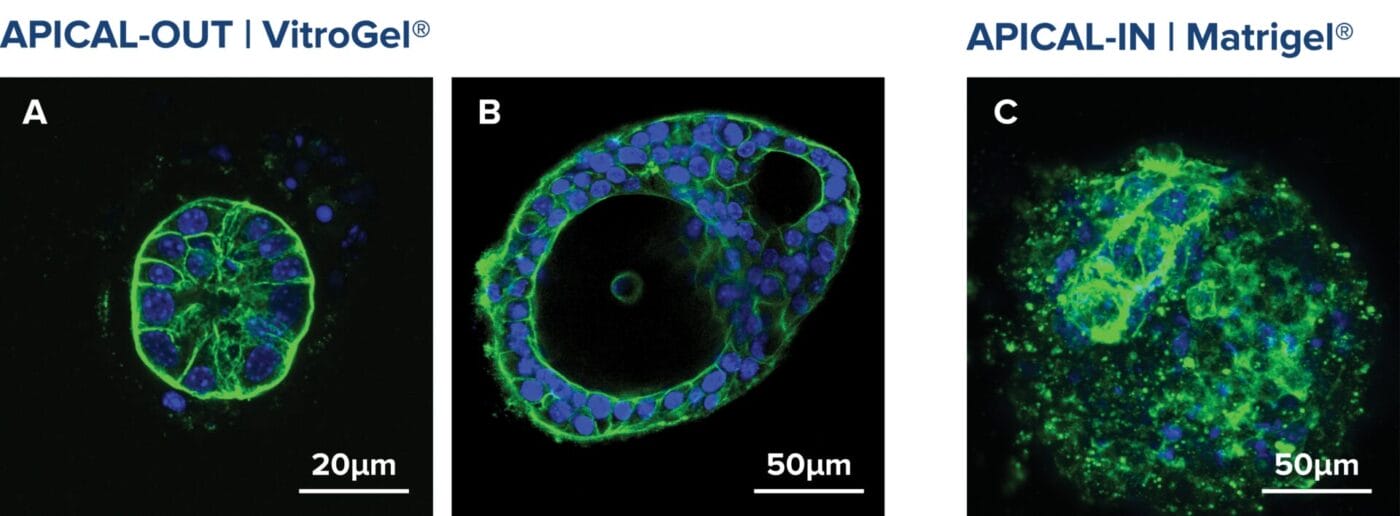
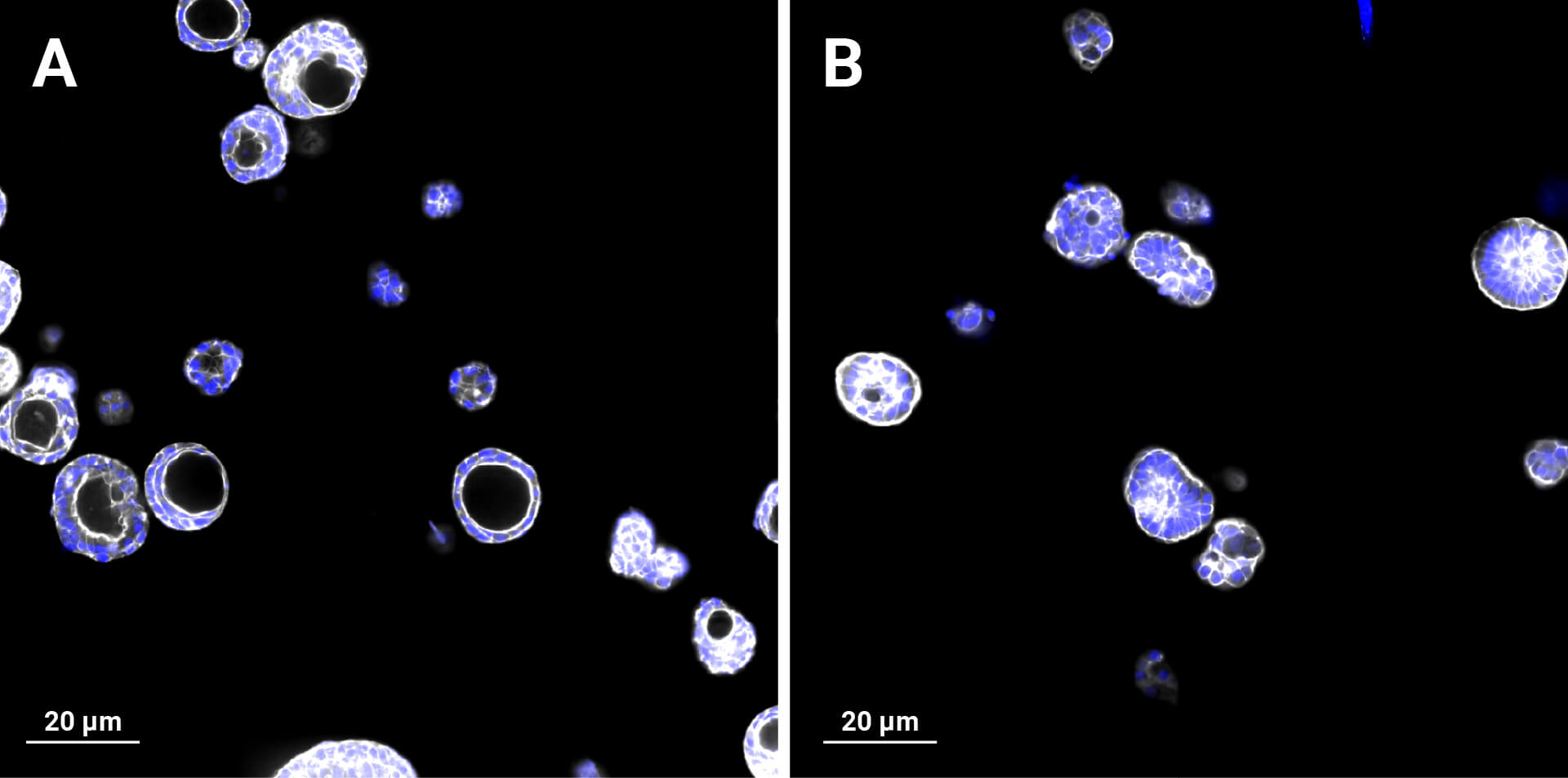
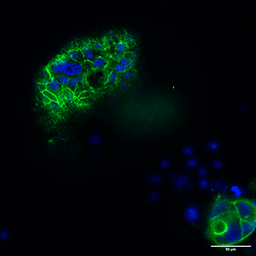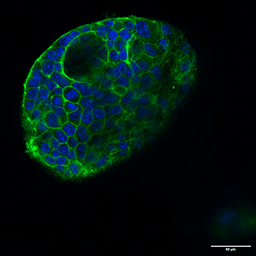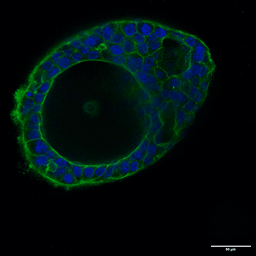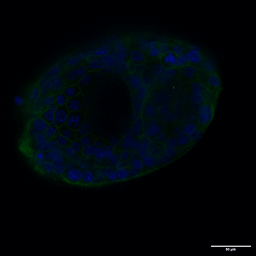
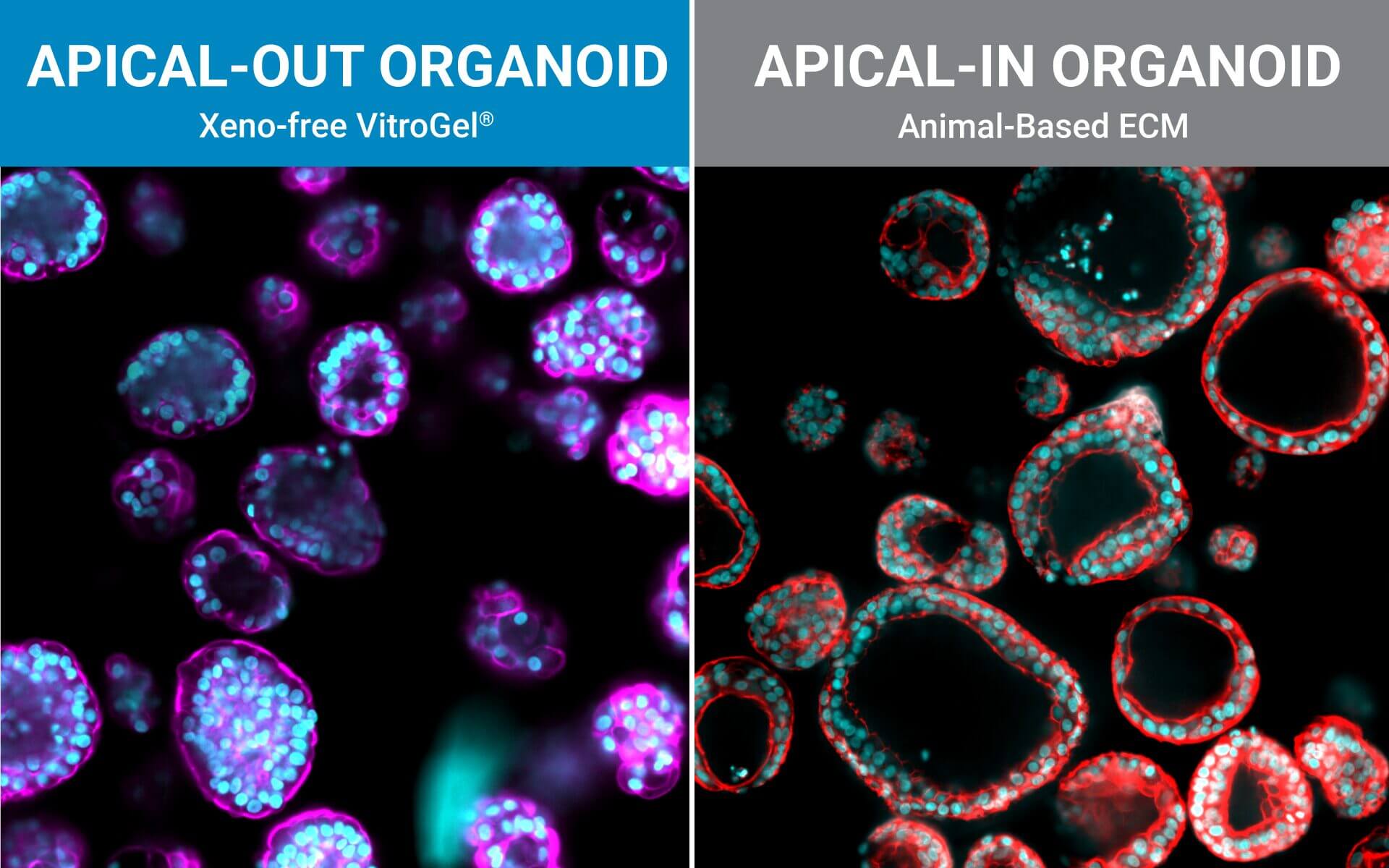
Several strategies exist: mechanical or enzymatic removal from the matrix, followed by suspension culture, can invert polarity (2). Although there is a higher probability of mixed polarity, diluted hydrogels can also promote apical-out formation. Each method has pros and cons regarding viability, reproducibility, and long-term maintenance. Importantly, recent reports indicate that some synthetic, chemically defined gels and controlled suspension strategies can produce stable apical-out organoids that maintain barrier function and transporter expression, enabling robust, repeatable experiments. For instance, with newer, innovative strategies, organoids can be cultured in an apical-out orientation using the synthetic hydrogel system VitroGel® ORGANOID. Both intestinal (Figure 3) and lung (Figure 4) organoids cultured in VitroGel® ORGANOID demonstrated apical-out polarity, unlike the apical-in polarity observed in animal-based hydrogels such as Matrigel.
Figure 3. VitroGel® ORGANOID supports apical-out polarity in intestinal organoids, in contrast to the apical-in polarity observed in animal-based hydrogels.
A. A young intestinal organoid with apical-out polarity cultured in VitroGel® ORGANOID 3 with RocketCell™ Intestinal Organoid Growth Kit. B. A mature intestinal organoid culture in VitroGel® ORGANOID 3 demonstrates complex lumen structures while maintaining apical-out polarity. C. An organoid with apical-in polarity culture in Matrigel with RocketCell™ Intestinal Organoid Growth Kit. Green represents Phalloidin staining and blue represents DAPI nuclei staining.
Figure 4. VitroGel® ORGANOID supports apical-out polarity in lung organoids, in contrast to the apical-in polarity observed in animal-based hydrogels. A. Lung organoids with apical-in polarity cultured in Matrigel. B. Lung organoids cultured in VitroGel® ORGANOID 3 with apical-out polarity. White represents Phalloidin staining and blue represents DAPI nuclei staining.
Practical Takeaways for Experimental Design
- Define the biological question first. If you need to expose the apical membrane to nutrients, drugs, microbes, or oral compounds, apical-out is usually the right choice. If you need luminal gradients or epithelial-ECM signaling, apical-in may be better (1).
- Choose protocols that preserve viability and phenotype. Some polarity-switch methods stress organoids; pick validated protocols that maintain junctional proteins and transporters (4). Synthetic VitroGel® ORGANOID hydrogel would be a better alternative to avoid such limitations.
- Validate polarity and function. Don’t assume orientation from appearance — confirm apical markers (e.g., villin, brush-border enzymes, Phalloidin: F-actin marker), tight junctions (ZO-1), and appropriate transporter localization. Functional assays (dye exclusion, uptake assays) are essential (5).
- Consider hybrid workflows. In some projects, it makes sense to expand organoids in one polarity and then transfer them to the other for specific assays; emerging studies show that organoids can adapt to new matrices and orientations if handled carefully (6).
Final Thoughts
The recent advances in nutrition review lay out how organoids are transforming nutrition research (1); adding polarity awareness to that toolbox ensures we ask the right questions and design the right experiments. Polarity is a simple phrase that masks an experiment-shaping choice. As organoid methods become central to nutrition science, drug discovery and translational work, explicitly considering whether your model is apical-in or apical-out and choosing the orientation that matches the biology you want to probe will improve reproducibility, interpretability, and translational relevance.
Our Commitment — Xeno-Free Solutions at TheWell Bioscience
VitroGel® ORGANOID is a xeno-free, synthetic hydrogel system designed for 3D organoid culture. Not only apical-out polarity, but it also supports long-term growth, and compatibility with a wide range of organoid types and culture methods, providing a reproducible and clinically relevant alternative to animal-derived matrices like Matrigel.
Learn moreAdditionally, this complete xeno-free system, including VitroGel® ORGANOID and the RocketCell™ Intestinal Organoid Growth Kit (TheWell Bioscience Cat # RC03-GK), supports the growth, expansion, and passaging of intestinal organoids while maintaining their natural apical-out structure throughout culture.
Learn moreReferences
- Menikdiwela, K. R., Lenis, M. J., & Storch, J. (2025). The Use of Organoid Cultures in Advancing Nutrition Research. Advances in nutrition (Bethesda, Md.), 16(10), 100489. https://doi.org/10.1016/j.advnut.2025.100489
- Co, Julia Y, et al. “Controlling the Polarity of Human Gastrointestinal Organoids to Investigate Epithelial Biology and Infectious Diseases.” Nature Protocols, vol. 16, no. 11, 18 Oct. 2021, pp. 5171–5192, https://doi.org/10.1038/s41596-021-00607-0.
- Tan, B., Yatim, S. M. J. M., Peng, S., Gunaratne, J., Hunziker, W., & Ludwig, A. (2020). The Mammalian Crumbs Complex Defines a Distinct Polarity Domain Apical of Epithelial Tight Junctions. Current biology : CB, 30(14), 2791–2804.e6. https://doi.org/10.1016/j.cub.2020.05.032
- Kakni, P., López-Iglesias, C., Truckenmüller, R., Habibović, P., & Giselbrecht, S. (2023). PSC-derived intestinal organoids with apical-out orientation as a tool to study nutrient uptake, drug absorption and metabolism. Frontiers in molecular biosciences, 10, 1102209. https://doi.org/10.3389/fmolb.2023.1102209
- Zietek, T., Giesbertz, P., Ewers, M., Reichart, F., Weinmüller, M., Urbauer, E., Haller, D., Demir, I. E., Ceyhan, G. O., Kessler, H., & Rath, E. (2020). Organoids to Study Intestinal Nutrient Transport, Drug Uptake and Metabolism – Update to the Human Model and Expansion of Applications. Frontiers in bioengineering and biotechnology, 8, 577656. https://doi.org/10.3389/fbioe.2020.577656
- Lee, M. G., Lee, B. R., Lee, P., Choi, S., Kim, J. H., Oh, M. H., & Yoo, J. G. (2024). Apical-out intestinal organoids as an alternative model for evaluating deoxynivalenol toxicity and Lactobacillus detoxification in bovine. Scientific reports, 14(1), 31373. https://doi.org/10.1038/s41598-024-82928-0
Discover how VitroGel® can support your cell culture research
Explore our updated resources for a concise overview of VitroGel®’s key features and application areas.
Comparison of VitroGel® vs. Animal-based ECM
Discover the 20+ advantages of VitroGel® over animal-based ECM with this comprehensive comparison on key features, operation, application, and storage conditions.
Learn MoreWhat is VitroGel® | Overview Video
Learn why VitroGel® is the leading animal-free hydrogel for 3D cell culture.
Learn MoreVitroGel® for Organoid Research
Learn how to achieve consistent organoid formation with VitroGel®, a fully synthetic, xeno-free hydrogel designed to support consistent, scalable organoid culture from stem cells or patient-derived tissues.
Learn More