Blog
John Huang Ph.D.
TheWell Bioscience Inc., Monmouth Junction, NJ

In April 2025, the U.S. Food and Drug Administration (FDA) announced a groundbreaking initiative to phase out the animal testing requirement for monoclonal antibodies and other drugs. This marked a significant milestone in the advancement of preclinical research. Experts from regulatory agencies, academia, and industry worldwide convened to promote the adoption of New Approach Methodologies (NAMs)—innovative alternatives, including organoids, microphysiological systems, and AI-driven models. These technologies aim to replace animal testing with more ethical, scalable, and human-relevant systems.
On July 7, 2025, the FDA, in collaboration with the National Institutes of Health (NIH), hosted a workshop on Reducing Animal Testing. FDA leaders reiterated the agency’s strategic roadmap to phase out most routine animal studies in favor of methods that offer superior reproducibility, predictive value, and mechanistic insight. The central message was clear: to truly transform the drug development process, we must not only replace animal models but also reconsider all legacy tools rooted in animal-derived components. During the workshop, the NIH also announced it would no longer fund studies that rely solely on animal testing, urging researchers to adopt NAMs.
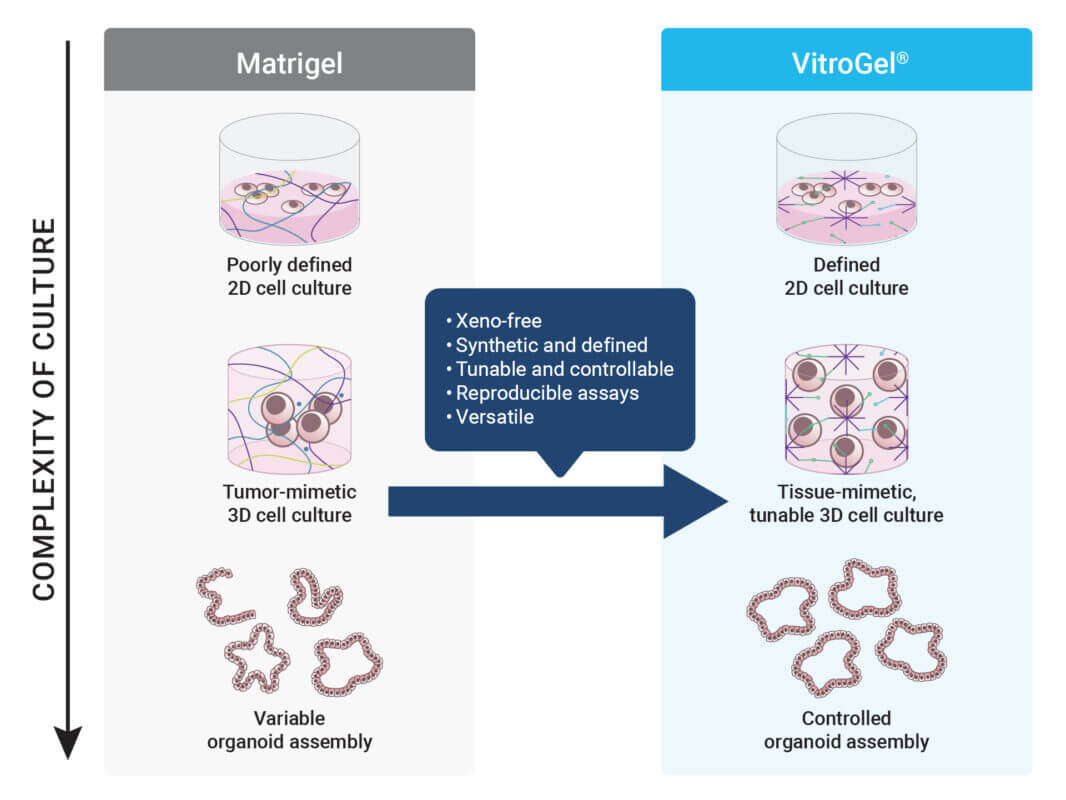
Despite this momentum, it is essential to sincerely evaluate a critical aspect that could impede the full realization of NAMs. Many next-generation cell and tissue models, particularly organoids and advanced 3D models, are still cultivated in animal-derived extracellular matrices (ECMs) such as Matrigel®. These materials, while historically useful, may no longer align with the ethical and scientific expectations that NAMs represent. These tumor-extracted materials stand in stark contrast to the principles underpinning NAMs. If the goal is to reduce and ultimately eliminate animal use, then continuing to rely on ECMs harvested from live animals undercuts both the ethical and scientific rationale behind NAM adoption.
The Ethical Conflict — Killing Animals to Replace Animal Testing
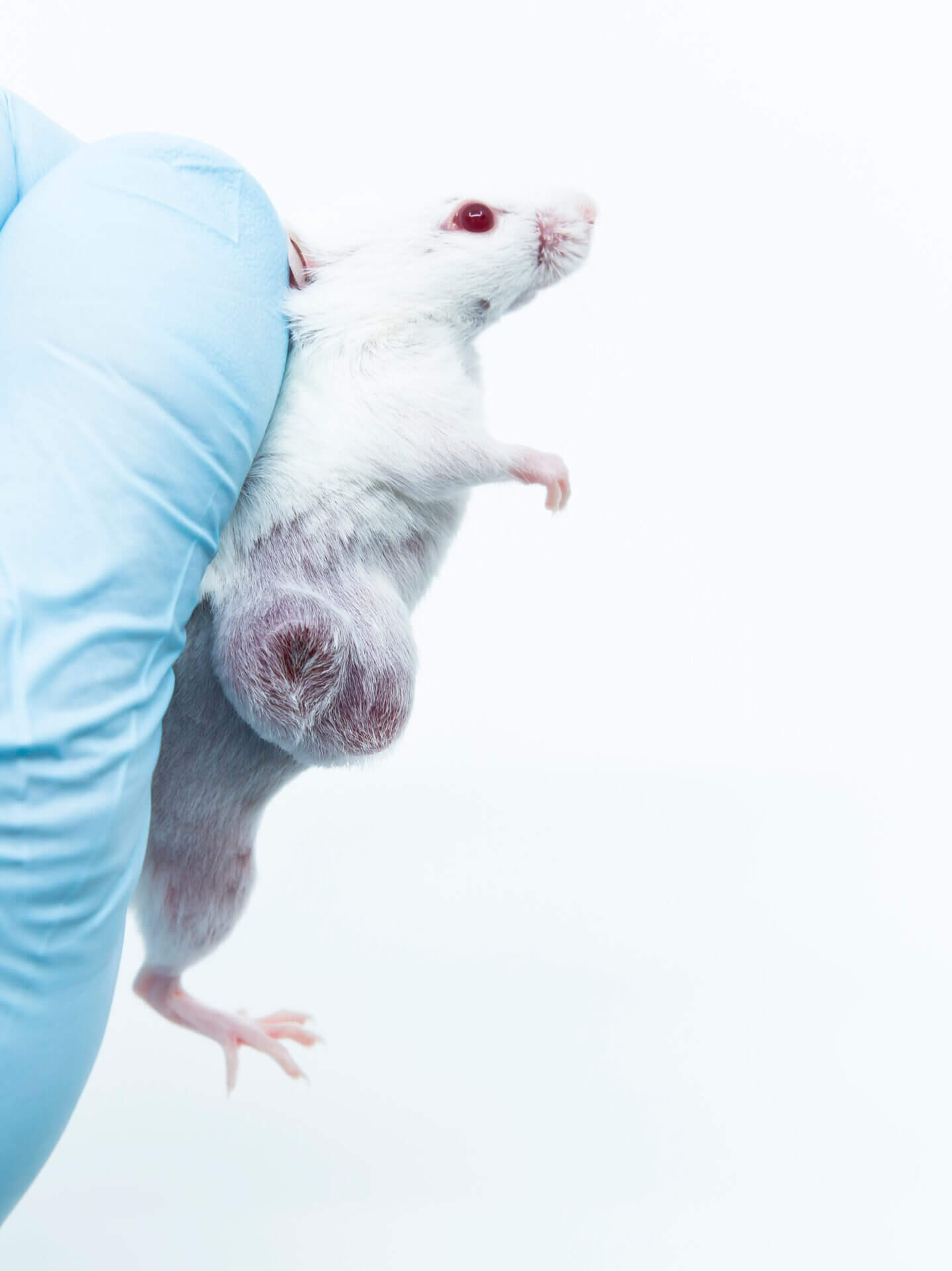
At the heart of this contradiction lies the production process of Matrigel®, a basement membrane extract obtained from Engelbreth-Holm-Swarm (EHS) mouse sarcomas. To generate a single commercial vial, suppliers typically sacrifice dozens of tumor-bearing mice. Globally, the Matrigel® supply chain is estimated to consume millions of mice per year.
These mice are not simply euthanized—they are grown with aggressive tumors that often exceed 10 cm³, well beyond ethical endpoints defined by oversight bodies. The animals endure significant suffering, including impaired mobility, cachexia, and systemic pain. This level of harm fundamentally contradicts the 3Rs principle (Replacement, Reduction, and Refinement) that underlies modern research ethics and regulatory guidance.
This ethical consideration deserves thoughtful attention. While the broader field continues to progress toward reducing animal use, the continued reliance on animal-derived reagents—such as basement membrane extracts sourced from tumor-bearing mice—raises important questions of alignment. For companies and institutions committed to the 3Rs and working to meet evolving expectations for humane, reproducible, and transparent science, addressing the use of animal-based extracellular matrix (ECM) is not just a scientific or regulatory concern—it may also pose a reputational risk if left unexamined.
The Scientific Flaws — Undefined Composition, High Variability and Misleading Data
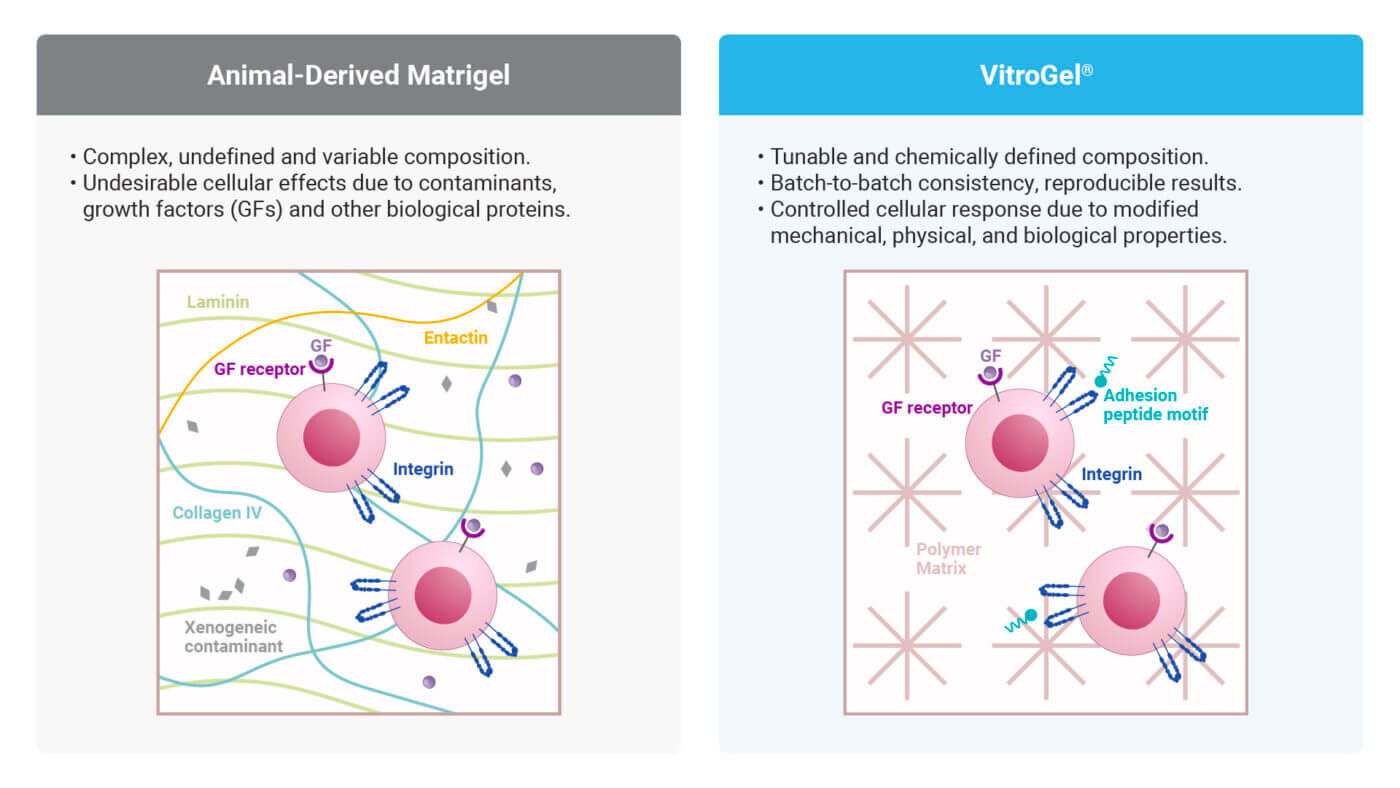
Beyond ethical issues, animal-derived ECMs are fraught with scientific limitations. These matrices are biologically complex but chemically undefined, introducing high variability and unpredictability into experimental systems. Every lot of Matrigel® is a heterogeneous mix of proteins, growth factors, and other tumor-derived components whose concentration and bioactivity can vary significantly.
Moreover, Matrigel® contains biologically active growth factors such as TGF-β, EGF, and FGF, which are known to trigger unwanted cellular programs, including epithelial-to-mesenchymal transition (EMT) and abnormal proliferation. For cancer or stem cell studies, such contamination can distort mechanistic insights and mislead therapeutic assessments. These limitations are not trivial. Inconsistent responses have been observed in drug screening studies, particularly in cancer research, where Matrigel-based models have been shown to overestimate tumor sensitivity to targeted therapies. Such discrepancies are thought to contribute to the high failure rate of oncology drugs in Phase II trials, partly due to inflated efficacy predictions from preclinical models that fail to translate clinically.
The Technical Barriers — Poor Compatibility with High-Throughput Screening and Automation
Additionally, animal-based ECMs present practical limitations that hinder scalability in drug discovery pipelines. One of the most significant challenges is their temperature sensitivity. Matrigel must be handled at 4°C to remain in a liquid form; it quickly gels at room temperature, making it difficult to use with liquid handling robotics and automated systems.
This handling complexity limits reproducibility, introduces variability in pipetting volumes, and complicates standardization across high-throughput workflows. As pharmaceutical and biotech companies increasingly adopt automation and high-throughput 3D screening to accelerate development timelines, the physical limitations of animal-based ECMs are becoming a critical bottleneck.
The Regulatory Landscape — NAMs Demand Reproducibility & Traceability
The workshop made one point especially clear: the future of preclinical science lies in methods that are defined, traceable, and human-relevant. Regulators emphasized the importance of replacing biologically ambiguous materials with well-characterized, scalable alternatives. Synthetic hydrogels—engineered with controlled mechanical properties, biological functions, and degradability profiles—meet these expectations. As preclinical testing becomes more data-driven and less reliant on animals, tools that ensure consistency across lots and labs will be indispensable. The shift is not just philosophical—it is structural, and institutions that fail to adapt will face growing scrutiny.
Our Commitment — Synthetic Biofunctional Hydrogels at TheWell Bioscience
At TheWell Bioscience, we are committed to building the foundation for next-generation, animal-free research. Our VitroGel® platform is a fully synthetic, biofunctional hydrogel system designed for advanced 3D cell culture, including organoid formation, stem cell expansion, cancer modeling, and high-throughput drug screening.
- Tunability – Precisely tunable matrix properties (e.g., stiffness, degradability, peptide motifs).
- Room Temperature Stable – Stays liquid at room temperature for easy handling. No ice bucket needed, with a simple and convenient protocol.
- Xeno-Free – 100% synthetic and free from animal or human-derived components. Supports diverse cell activities and is safe for in vivo applications.
- Lot-to-lot consistency – Ensures reproducible results across experiments and laboratories.
- Injectable – Maintains long-term injectability after gelation without needle clogging. Its unique shear-thinning property makes it ideal for xenografts, cell therapy, and drug delivery applications.
Our goal is to empower researchers to achieve more reliable, ethical, and future-ready science. In eliminating animal-derived ECMs, we not only enhance scientific accuracy—we uphold the core values driving the transformation of biomedical research.
Discover how VitroGel® can support your cell culture research
Explore our updated resources for a concise overview of VitroGel®’s key features and application areas.
Comparison of VitroGel® vs. Animal-based ECM
Discover the 20+ advantages of VitroGel® over animal-based ECM with this comprehensive comparison on key features, operation, application, and storage conditions.
Learn MoreWhat is VitroGel® | Overview Video
Learn why VitroGel® is the leading animal-free hydrogel for 3D cell culture.
Learn MoreVitroGel® for Organoid Research
Learn how to achieve consistent organoid formation with VitroGel®, a fully synthetic, xeno-free hydrogel designed to support consistent, scalable organoid culture from stem cells or patient-derived tissues.
Learn More