bFGF/FGF-2 (Human), Recombinant Protein, E. coli, Tag Free
Premium CytoGrow™ growth factor suitable for stem cell culture, angiogenesis research, wound healing studies, and regenerative medicine.
CytoGrow™ Recombinant bFGF/FGF-2 (Human), E. coli, Tag Free
| Synonym: | Fibroblast growth factor 2; FGF-2; Basic fibroblast growth factor; bFGF |
| Species: | Human |
| Expression System: | E. coli |
| Endotoxin: | ≤0.1 EU/mg |
| Appearance: | White powder, colorless liquid after reconstitution |
| Formulation | Lyophilized from a 0.22 μm filtered solution containing PBS, 5% mannitol, and 0.01% Tween 80 |
| Reconstitution: | Recommended to redissolve in sterile deionized water |
| Shipping: | US - Ships overnight |
| Storage and Stability: | Store in a manual defrost freezer and avoid repeated freeze-thaw cycles. Lyophilized state: 36 months at -20°C to -80°C After reconstitution and under sterile conditions: 6 months at -20°C to -80°C 7-10 days at 2°C to 8°C |
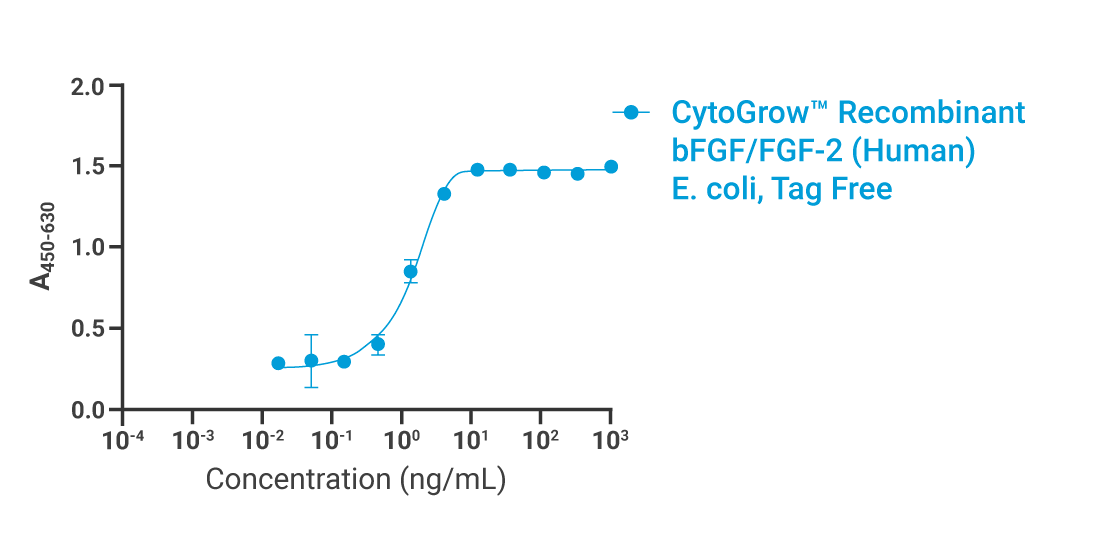
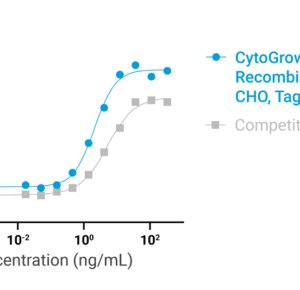
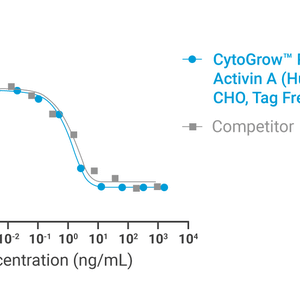
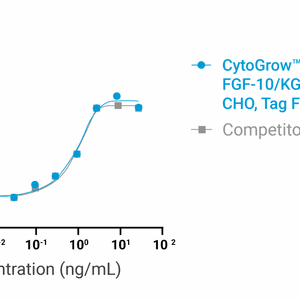
Bioactivity
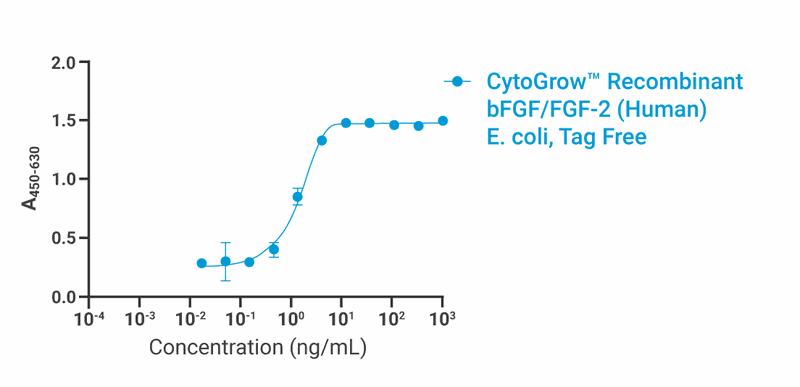
Data

Neuron Culture
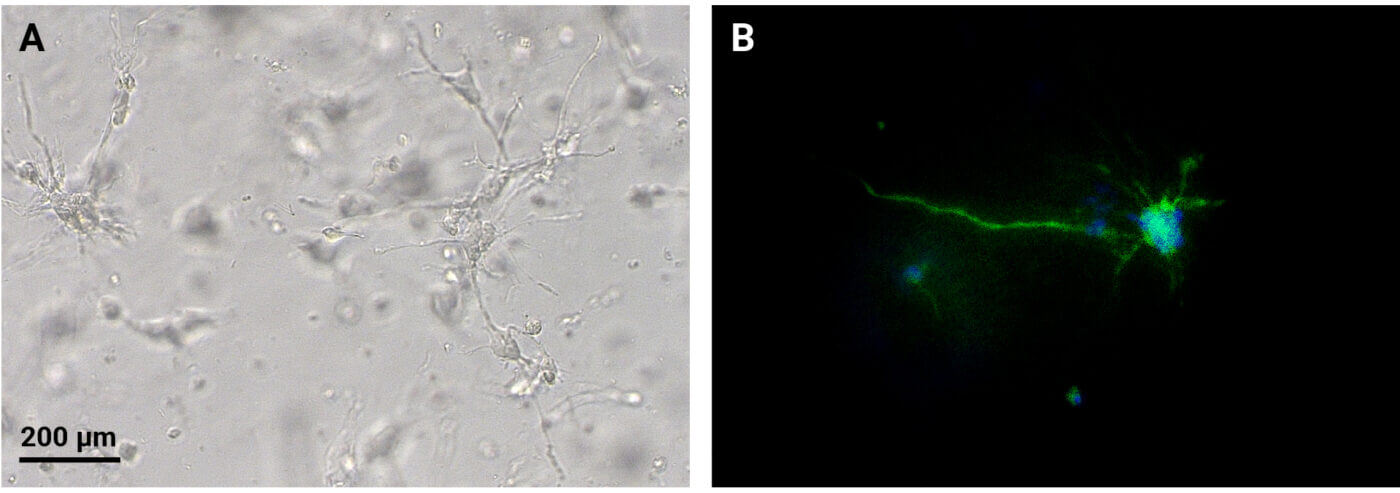
Figure 1. CytoGrow™ growth factors support 3D encapsulation culture of NSC cells as single cell suspension in VitroGel® NEURON using RocketCell™ NSC Xeno-Free Growth Medium.
VitroGel® NEURON Hydrogel and RocketCell™ NSC Xeno-Free Media Support 3D NSC Cultures and Neurite Outgrowth. The growth medium was supplemented with CytoGrow EGF and FGF2. 3D NSC cultures were established by preparing a single-cell NSC suspension with RocketCell™ NSC Xeno-Free Medium and mixing with VitroGel NEURON for 6 days (A). Immunofluorescence staining was performed to evaluate the presence of beta-III-tubulin, a neuron maturation marker. The images were obtained using the Keyence BZ-X microscope at 10x and 20x magnifications (B).
Figure 2. CytoGrow™ growth factors support NSC growth and maintenance on VitroGel® NEURON coating surface of VitroPrime™ Spread-Attach using RocketCell™ NSC Xeno-Free Growth Medium.
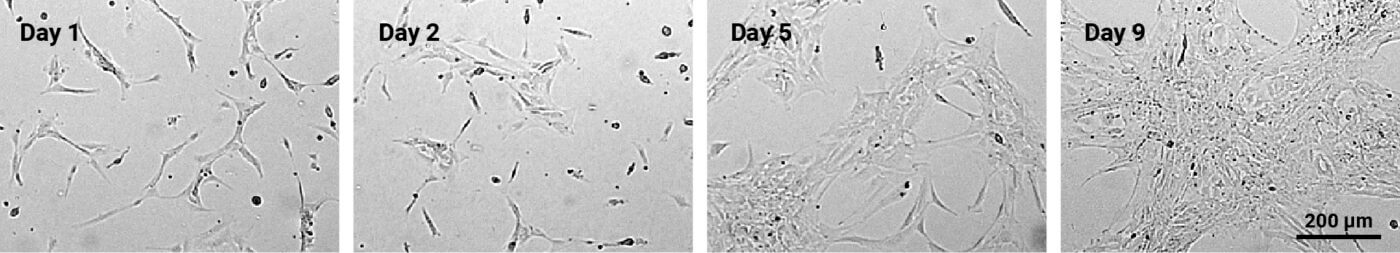
24-well plates were coated with VitroGel® NEURON hydrogel (1:200 dilution) for 30 minutes at room temperature. iPSC-derived NSCs (4 x 104 cells/well) were added to each well, resulting in a final volume of 0.5 mL per well. The medium was changed every 2 days, and the cultures were monitored using an inverted microscope at a 10X magnification.
Stem Cell Culture
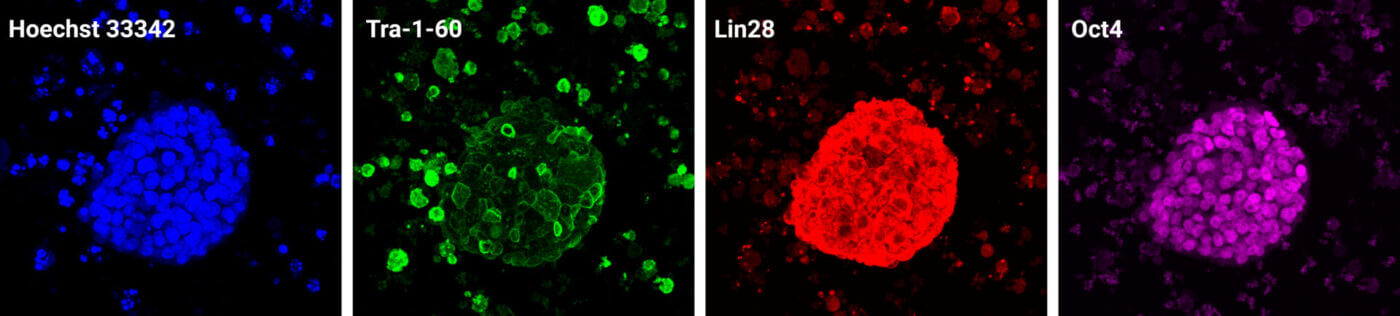
Figure 3. Confocal imaging of IPSC spheroids growing in VitroGel® STEM.
IPSC cultures were plated in VitroGel® STEM and grown for 5 days. The slides were imaged using a Leica Mica confocal microscope. An IPSC spheroid was imaged in 3D using the Z-stacking function, and images for Hoechst 33342 (DNA), Tra-1-60 (Podocalyxin, cell surface), Lin28A (cytoplasmic RNA binding protein), and Oct4 (nuclear transcription factor) were projected using the maximum intensity algorithm.
| Size | 1 μg, 10 µg, 50 μg, 1 mg, 100 mg, 1 g, 1 mL, 10 mL |
|---|