White Papers
3D Cell Culture Scale-up by Using VitroGel® Hydrogel Matrix embedding in CelVivo ClinoReactor™


WHITE PAPER
TheWell Bioscience Inc.
1 Deerpark Dr, Ste C, Monmouth Junction, NJ 08852, USA
CelVivo
Ørbækvej 264, 5220 Odense, Denmark
Introduction
Cells exhibit diverse behaviors influenced by their morphological and functional characteristics. While some cells can naturally self-assemble into three-dimensional (3D) structures from a suspension of single cells, others require additional steps — such as encapsulation — to achieve similar constructs. Moreover, some cell types produce excessive extracellular matrix (ECM) proteins, leading to uncontrolled aggregation and fusion, whereas others struggle to generate sufficient ECM, hindering autonomous 3D formation.
VitroGel®, a xeno-free synthetic ECM substitute, provides a robust and reproducible solution to overcome these challenges. By embedding cells within VitroGel®, researchers can precisely control the microenvironment, ensuring uniform aggregate formation while maintaining high cell viability and yield. The CelVivo ClinoReactor™ is a rotating bioreactor vessel designed to enable the formation of physiologically relevant 3D constructs. When placed in the ClinoStar® system, the reactor maintains gentle, continuous rotation that minimizes shear stress and supports long-term vertical culture. By combining these two technologies, researchers can generate a desired microenvironment in a controlled dynamic culture that fosters 3D tissue-like organization. This white paper details the integration of the synthetic, versatile, biofunctional VitroGel® Hydrogel Matrix (TheWell Bioscience, Catalog #: VHM01) with the CelVivo ClinoReactor™ inside the ClinoStar® system to establish a standardized, scalable, and reproducible 3D culture method.
Materials
1. Cells (e.g. MDA-MB-231 cells (HTB-26, ATCC))
2. Growth media
3. VitroGel® Hydrogel Matrix (VHM01, TheWell Bioscience)
4. ClinoReactor™ (CelVivo)
5. ClinoStar® (CelVivo)
6. Single channel electronic pipette in multiple dispensing
mode (Sartorius eLINE, 0.2-10 μL)
7. Gel loading tip (F1731281, Pipetman)
8. Syringe (B.Braun, 4616103)
9. Needle (B.Braun, 466512)
Methods
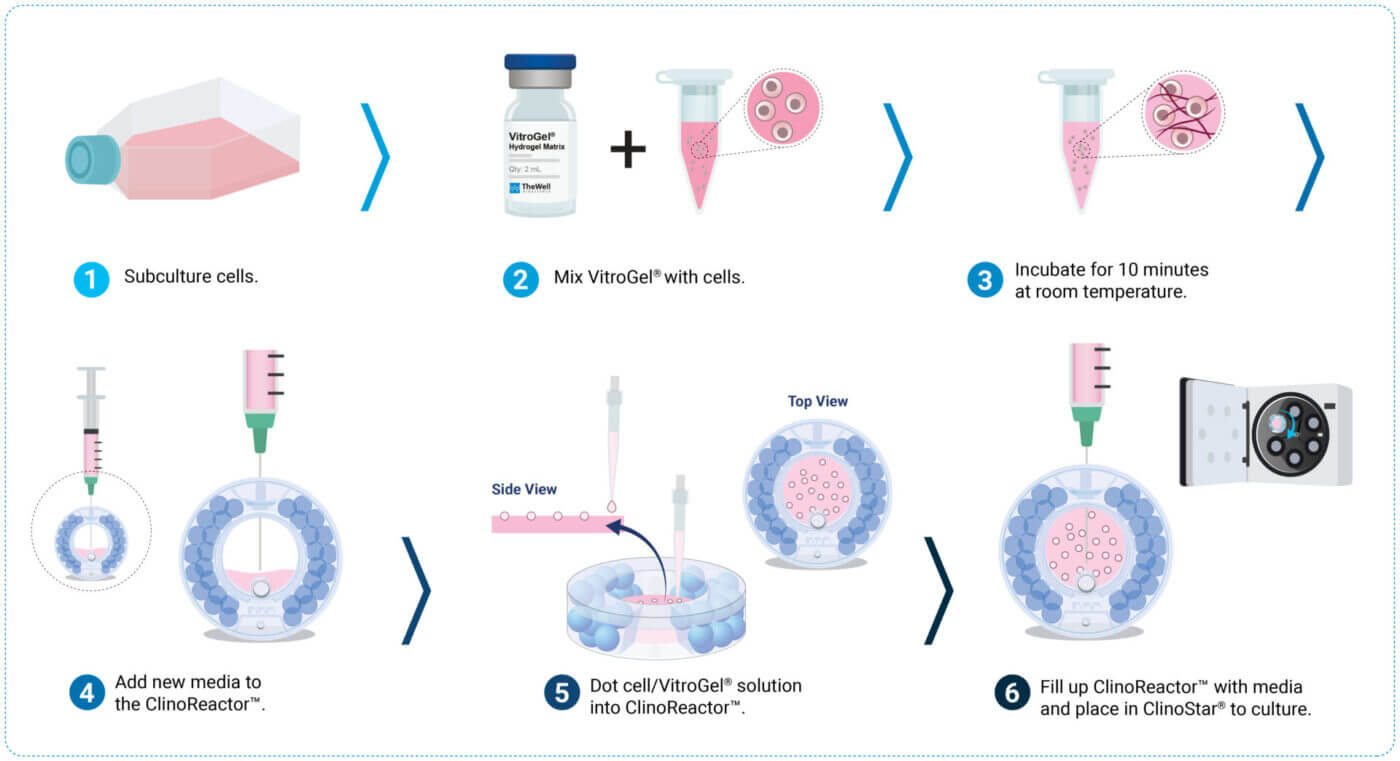
Preparation
- Hydrate and equilibrate the appropriate amount of ClinoReactor™(s) according to CelVivo protocol 0031 or Wrzesinski et al.2.
- Allow VitroGel® Hydrogel Matrix to reach room temperature.
- Follow the manufacturer’s protocols/guidelines to subculture cells.
Preparation of VitroGel®/Cell Suspension
- Transfer 1.5 million cells to a 15 mL Falcon tube. (See Note 1 for cell concentration range.)
- Centrifuge the cell solution in a Falcon tube at 140 g for 5 minutes.
- Aspirate supernatant.
- Add 100 μL of fresh media to the cell pellet.
- Subsequently, add 200 μL VitroGel® Hydrogel Matrix to the cell solution and mix well (pipetting up and down ~10 times), creating a cell solution with 5,000 cells/μL. Avoid creating bubbles.
- Incubate the VitroGel®/cell solution for 10 minutes at room temperature.
ClinoReactor™ Set up
- Remove equilibration liquid from ClinoReactor™ vessel.
- Add 5 mL fresh growth media to the cell chamber of ClinoReactor™ vessel and position it in petri dish mode (flat on the back with open front lid).
Dotting of VitroGel® Hydrogel Matrix into the ClinoReactor™
- Adjust the single-channel electronic pipette in multiple dispensing mode to 1 μL with a total of 10 μL.
- Dot VitroGel®/cell solution into the cell chamber of ClinoReactor™ using a gel loading tip. Dispense 1 μL of the solution as a droplet at the end of the tip. Carefully touch the droplet with the surface of the media in the ClinoReactor™ to release the droplet (see Figure 2A).
- Repeat step 2 until 60-100 VitroGel®/cell dots are created. For suggestions on troubleshooting, see Note 2.
Initiating the Culture
- Swirl the ClinoReactor™, which forces dots to be submerged in media.
- Check for irregular shape dots and remove them from the cell chamber.
- Close the lid of the ClinoReactor™ vessel and position it in an upright position.
- Fill the ClinoReactor™ with growth media as described in CelVivo protocol 0041 or Wrzesinski et al.2.
- Position the ClinoReactor™ in the ClinoStar® and start culture at 15 rpm.
- Adjust speed accordingly, and if constructs stick together, see Note 3.
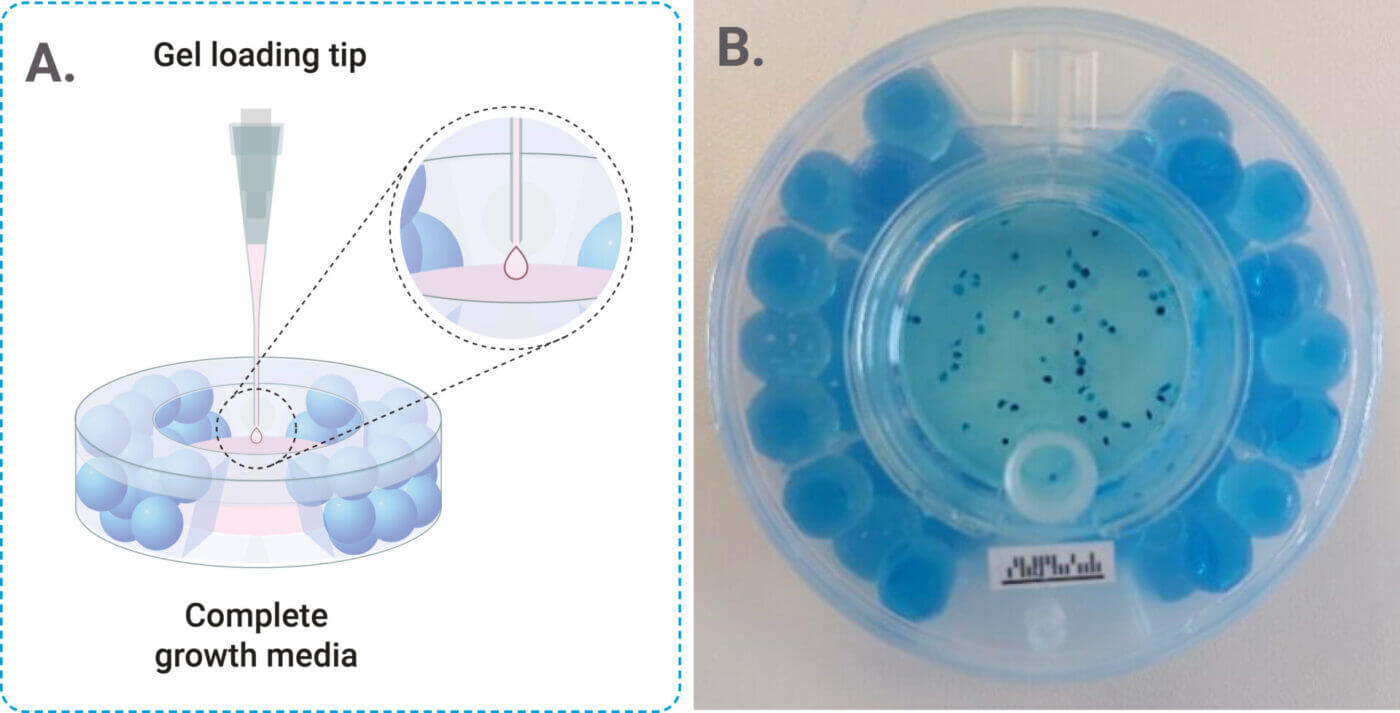
Figure 2. A) Representative image of the Dotting Process. B) Representative image of ClinoReactor™ set up with VitroGel® system.
Note 1: Cell concentrations can be made ranging from 600 to 10,000 cells per μL dot.
Note 2: For easier troubleshooting, dissolve E133 dye (e.g., 861146-5G, Sigma Aldrich) in a VitroGel® aliquot, followed by vortexing and filter sterilisation. Then resuspend cells in dyed sterile VitroGel® and complete the incubation time at room temperature before VitroGel® is ready to use for dotting. The E133 dye stains the otherwise translucent dots blue, facilitating easy handling in colorless or red-colored media. The blue dye will dilute out in approximately 2 days.
Note 3: During the first days of culture, the construct may appear sticky and clump together. This is due to the presence of overdiluted VitroGel® on the surface of the construct. Separate the spheroids using the ClinoStar® disperse function. If they still stick together, take the ClinoReactor™ out of the ClinoStar® and rotate the ClinoReactor™ manually first one way and then the other. Increase force if needed. The “stickiness” disappears after a few media changes.
Results and Discussion
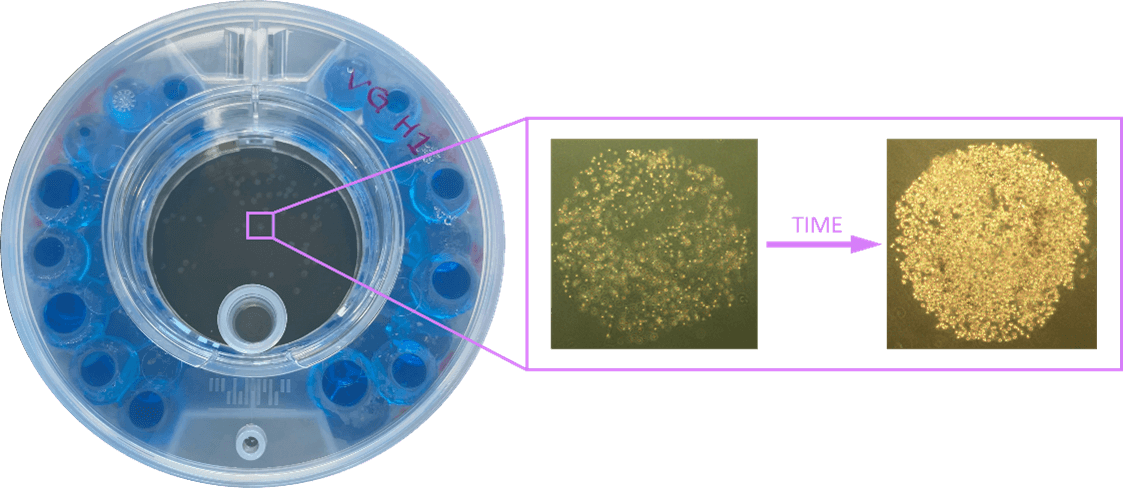
MDA-MB-231 cells were suspended in cell culture medium and then mixed with VitroGel® Hydrogel Matrix according to the manufacturer’s instructions. The hydrogel-cell dotting was easily done directly into the ClinoReactor™. Within 24 hours, MDA-MB-231 cells formed consistent, 3D constructs supported by the hydrogel matrix. These constructs displayed homogeneity in size and retained their morphology over an extended culture period of 21 days (See Figure 3A-D).
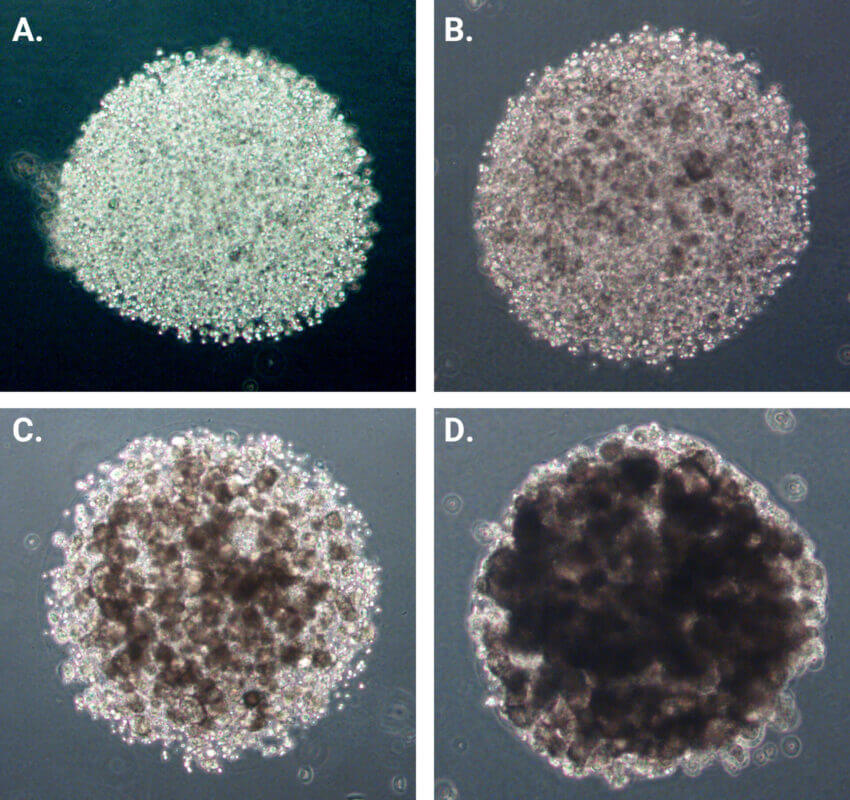
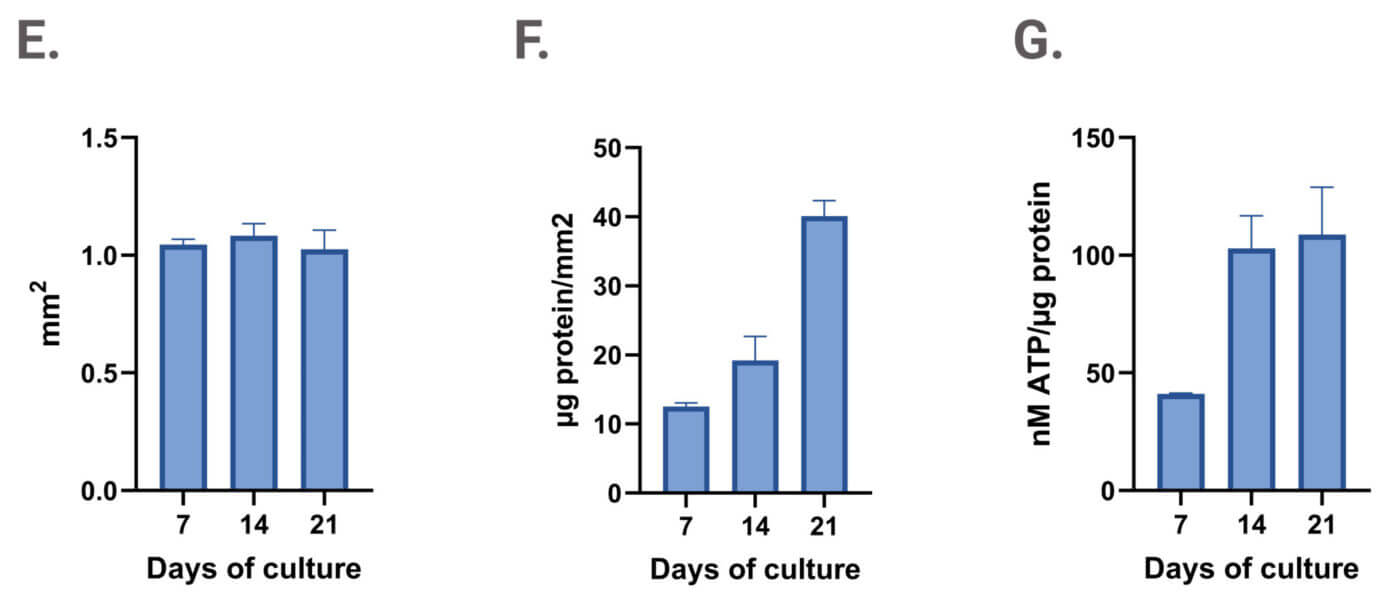
Figure 3. Dotting of VitroGel®/cell suspension in ClinoReactor™. Uniform droplets (1 μL each) were dispensed into the cell chamber and submerged by gentle swirling. Constructs were evenly distributed throughout the chamber. Images show a representative 3D construct at four time points: (A) Day 0 after encapsulation, (B) Day 7, (C) Day 14, and (D) Day 21. Planimetry (E), protein content normalized to area (F), and viability normalized to biomass (G) of VitroGel® drops cultured in ClinoReactor.
The use of VitroGel® in combination with the ClinoStar® system offers key advantages, including standardized protocols and stable constructs that remain suspended and viable (see Figure 3G) without excessive aggregation. This setup supports growth, as evidenced by increased protein content per mm² (see Figure 3E and F).
Conclusion
The combination of VitroGel® Hydrogel Matrix with the CelVivo ClinoReactor™ offers a robust and standardized platform for advanced 3D cell culture. This integrated approach enables the reproducible embedding of cells within a physiologically relevant, xeno-free microenvironment that closely mimics in vivo conditions. As demonstrated, this system is highly consistent for generating reliable and translational in vitro models. The VitroGel®–ClinoReactor™ method delivers easy handling, reproducibility, and scalability, making it especially suitable for high-throughput applications and preclinical research.
References
- Celvivo online protocols library: https://celvivo.com/community/selected-protocols/